

Ideas and Innovation - Year 2018 - Volume 33 -
Histological evaluation of latissimus dorsi muscle subjected to tissue expansion after infiltration with botulinum toxin: an experimental study in rats
Avaliação histológica do músculo grande dorsal submetido à expansão tecidual pós-infiltração com toxina botulínica: estudo experimental em ratas
ABSTRACT
The authors describe histological changes in the latissimus dorsi muscle submitted to expansion after relaxation with botulinum toxin. The possible practical benefits include increased muscle compliance and better accommodation of a prosthesis. The experimental model involved 10 Wistar rats (Rattus norvegicus) of the same age, with average weight of 300 g. Muscle biopsies before and after expansion were performed in normal muscle, in a control group (with expanders alone), and in a group with expanders and botulinum toxin. Expanders measuring 3 cm3 were positioned below the muscle and expanded with 0.3 ml of saline weekly, for 10 weeks. Histological sections were stained using hematoxylin-eosin for general evaluation and Masson's trichrome for evaluation of connective tissue. The muscle fibers submitted to expansion under the action of botulinum toxin showed less fibrosis and less intense proliferation of blood vessels than in the group without botulinum toxin, and the atrophy and reduction in the number of muscle fibers were less prominent than in the group that did not receive botulinum toxin. The findings suggest that muscle expansion associated with botulinum toxin relaxation preserves skeletal muscle characteristics by providing better accommodation and protection for a prosthesis and facilitating expansion dynamics; this method may also reduce pain.
Keywords: Tissue expansion devices; Botulinum toxin; Histology
RESUMO
Os autores descrevem as alterações histológicas no músculo grande dorsal submetido à expansão após relaxamento com toxina botulínica e as possíveis correlações dos achados com os benefícios práticos como, por exemplo, aumento da complacência muscular e melhor acomodação da prótese. Foi empregado o modelo experimental, com dez ratas com peso médio de 300 g, mesma faixa etária, da cepa Wistar (Rattus norvegicus) e o músculo grande dorsal. Biópsias musculares foram feitas antes e após as expansões, no músculo normal, no grupo controle (apenas com expansores) e no grupo com expansores e toxina botulínica. Expansores de 3 centímetros cúbicos eram posicionados abaixo do músculo e expandidos com 0,3 mililitros de soro fisiológico semanalmente, por 10 semanas. Os cortes histológicos foram corados segundo as técnicas de Hematoxilina-eosina, para avaliação geral, e tricrômio de Masson para avaliação do tecido conjuntivo. As fibras musculares submetidas à expansão sob a ação da toxina botulínica apresentaram focos de fibrose e proliferação de vasos sanguíneos menos intensos que no grupo sem toxina botulínica e a diminuição do número de fibras musculares e a atrofia eram menores que no grupo que não utilizou a toxina. Os achados nos permitem presumir que a expansão muscular associada ao relaxamento com toxina botulínica preserva as características da musculatura esquelética, oferecendo melhor acomodação e proteção da prótese e facilitando a dinâmica da expansão, além de diminuir a dor.
Palavras-chave: Dispositivos para expansão de tecidos; Toxinas botulínicas; Histologia
INTRODUCTION
Tissue expansion is widely used in plastic surgery and relies on the biomechanical properties of tissues submitted to progressive tension1.
Natural expansion with rapid adaptation occurs during physical growth, as in the enlargement of breasts at puberty and during gestation2.
The expansion process can be used in larger lesions or in areas with limited distensibility, allowing repair of large tissue losses of congenital origin or those caused by trauma or tumors3-5.
A major problem in progressive muscle expansion is pain, probably secondary to muscle spasm induced by hypoxia. Attenuation or absence of pain in elective or traumatic surgical denervation of a particular muscle that will be expanded is observed, for example, in breast reconstruction with use of an expandable prothesis6,7. In addition, pain is reduced after relaxation with botulinum toxin in the expanded muscle used for breast reconstruction with a prosthesis7,8.
Expansion leads to a gain in muscle compliance, contributing to decreased tension in the sutures. The histological changes include muscular hypertrophy, change in fiber shape and nuclear position, and an increase and changes in blood vessels. These changes are not seen in acute muscle expansion performed intraoperatively because a longer time is needed to stimulate permanent muscle growth4.
OBJECTIVES
This study describes the histological changes in the latissimus dorsi muscle submitted to expansion after relaxation with botulinum toxin. Potential practical benefits include increased muscle compliance, better accommodation of a prosthesis, and reduction of pain.
METHODS
This is the pilot study for a research project approved by the Ethics Committee on the Institutional Use of Animals. Ten Wistar rats (Rattus norvegicus) with a mean weight of 300 g and age about 6 months were used. The muscle used for expansion was the latissimus dorsi because it was easy to approach and thin, with a visible cleavage plane and underlying bone.
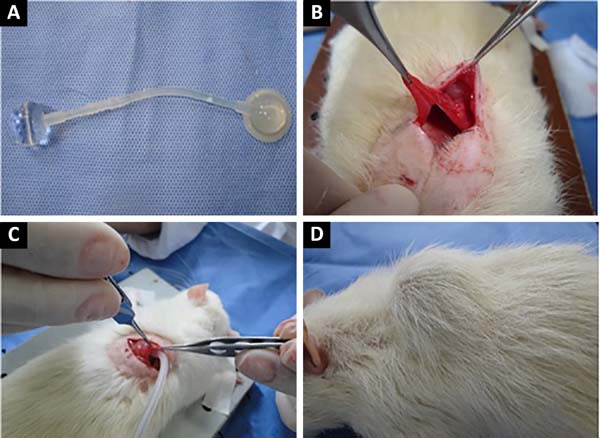
Human orbit expanders with diameter of 1.7 cm and volume of 3 ml were used. The expanders were smooth, with two valves, and were sterilized with ethylene oxide. The expanders were registered at the National Agency of Sanitary Surveillance (Figure 1-A).
Botulinum toxin type A was used in lyophilized form. A 100 IU vial was diluted with 4 ml of 0.9% physiological solution to obtain 25 IU/ml at a dose of 1 IU/cm2 of muscle. Infiltration was performed with 1 ml syringes and insulin needles and two reference traces of the syringe infiltrated.
Five animals were submitted to expansion of the latissimus dorsi without botulinum toxin and 5 received prior botulinum toxin.
After incision and identification of the latissimus dorsi, submuscular blunt dissection was performed to create a space for the expander (Figure 1-B). The valve connected to the expander was positioned in subcutaneous tissue to facilitate identification for future puncture. Muscle samples were collected for histological evaluation before and at the end of the expansion. Ten expansions were made, at intervals of 7 days, using 0.3 ml for each treatment.
To insert the expander, the animals were anesthetized with 2% xylazine hydrochloride at a dose of 5 mg/kg, at a concentration of 9.1 mg/ml (weight × dose × 1/concentration), combined with ketamine at a dose of 50 mg/kg, intraperitoneal (Figure 1-C).
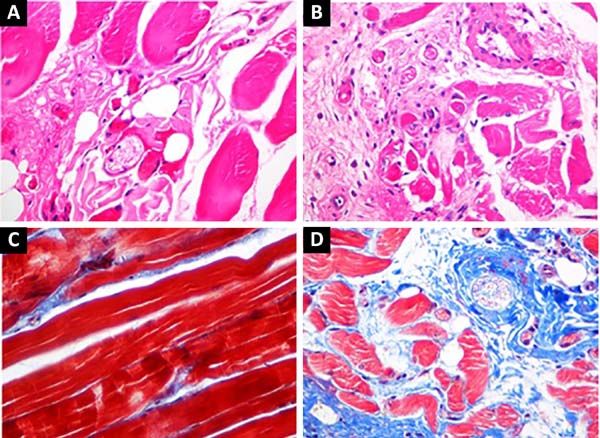
Immediately after positioning of the expander, 10% of its capacity was filled with methylene blue dye saline to monitor possible extravasation. After complete expansion (Figure 1-D), muscle samples were collected for histological analysis and immediately fixed in 4% buffered formalin. The samples were processed, embedded in paraffin, and 4-μm slices were prepared with a microtome. Histological sections were stained with hematoxylin-eosin (HE) for general evaluation, and Masson’s trichrome (TM) for evaluation of connective tissue.
The slides were simultaneously examined under a standard light microscope by 2 observers. The presence of muscle atrophy, change in the position and size of the muscle cell nucleus, presence of inflammation, change in the number of blood vessels, and the presence of deposits were evaluated in the samples stained with HE.
The findings were compared in the pre-expansion groups with and without prior relaxation with botulinum toxin. In the samples stained with TM, the amount of blue-stained connective tissue between the red-stained muscle fibers was evaluated.
RESULTS
Muscle fibers biopsied prior to expansion in animals from the groups with and without botulinum toxin relaxation were considered normal muscle and used as controls.
Animals submitted to expansion without botulinum toxin showed foci of fibrosis, with an increase in connective tissue between the muscle fibers and between the muscle fascicles. The changes were more intense than in the group that underwent relaxation with botulinum toxin.
In addition, there was a greater increase in the number of blood vessels, skeletal muscle atrophy, and centralization of nuclei, as well as less muscle. The group without botulinum toxin also exhibited foci of old hemorrhage and mild nonspecific inflammatory infiltrates with lymphocytes and plasma cells; these were not seen in the botulinum toxin group (Figure 2).
DISCUSSION
It is believed that the increased fibrosis in animals submitted to skeletal muscle expansion probably results from tissue ischemia caused by expansion and consequent distension and compression of blood vessels. Cytokines would be released and would induce vascular proliferation9.
When the botulinum toxin group was evaluated, fibrosis and vascular proliferation were less intense. Relaxation in the toxin-treated musculature probably decreased the local ischemia8,10-12, explaining the differences. In addition, less atrophy was found, as no inflammation was found, possibly due to decreased release of proinflammatory cytokines9. Easier expansion after muscle relaxation would explain the absence of bleeding in this group6,7.
A major problem in progressive tissue expansion is the pain that can lead to interruption of treatment8,13. Studies have demonstrated reduced pain in expanded muscle used in breast reconstruction, following prosthesis insertion with botulinum toxin pretreatment8,9,13.
In addition to muscle relaxation, it is believed that the decreased hypoxia in muscle fibers is associated with reduction of pain, perhaps due to a decrease in the release of pain-inducing substances6,13.
CONCLUSIONS
Botulinum is relatively safe and easy to use. Botulinum-induced muscle relaxation results in less abrupt and painful muscle stretching.
Because of the small size of the sample, further studies are needed. However, these findings agree with literature data, allowing us to assume that use of botulinum toxin-treated expanded skeletal muscle, in addition to reducing pain, allows greater relaxation and preservation of muscle tissue, with added distensibility and better accommodation of a prosthesis.
COLLABORATIONS
|
MPSN |
Conception and design of the study; completion of surgeries and/or experiments. |
|
LCR |
Conception and design of the study; completion of surgeries and/or experiments. |
|
ECSA |
Analysis and/or interpretation of data; final approval of the manuscript; writing the manuscript or critical review of its contents. |
|
ACRD |
Completion of surgeries and/or experiments; writing the manuscript or critical review of its contents. |
|
ACPB |
Analysis and/or interpretation of data; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents. |
|
RME |
Analysis and/or interpretation of data; final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents. |
REFERENCES
1. Argenta LC, Marks MW. Principles of tissues expansion. In: Mathes SJ, ed. Plastic surgery: general principles. Philadelphia: Saunders Elsevier; 2006. p. 539-67.
2. Neumanm CG. The expansion of an area of skin by progressive distention of a subcutaneous balloon: use of the method for securing skin for subtotal reconstruction of the ear. Plast Reconstr Surg (1946). 1957;19(2):124-30. DOI: http://dx.doi.org/10.1097/00006534-195702000-00004
3. Chun JT, Rohrich RJ. Versatility of tissue expansion in head and neck burn reconstruction. Ann Plast Surg. 1998;41(1):11-6. PMID: 9678462 DOI: http://dx.doi.org/10.1097/00000637-199807000-00003
4. De Filippo RE, Atala A. Stretch and growth: the molecular and physiologic influences of tissue expansion. Plast Reconstr Surg. 2002;109(7):2450-62. DOI: http://dx.doi.org/10.1097/00006534-200206000-00043
5. Gemperli R, Brechtbühl ER. Expansores teciduais. In: Mélega JM, ed. Cirurgia plástica: fundamentos e arte: princípios gerais. Rio de Janeiro: Medsi; 2002. p. 177-83.
6. Layeeque R, Hochberg J, Tillman R, Westbrook K, Yuen JC, Kunkel KM, et al. Botulinum toxin infiltration for pain control after mastectomy and subpectoral tissue expansion. Ann Surg 2004;240(4):608-14.
7. Lu L, Atchabahian A, Mackinnon SE, Hunter DA. Nerve injection injury with botulinum toxin. Plast Reconstr Surg. 1998;101(7):1875-80. PMID: 9623830 DOI: http://dx.doi.org/10.1097/00006534-199806000-00015
8. LoGiudice J, Gosain AK. Pediatric tissue expansion: indications and complications. J Craniofac Surg. 2003;14(6):866-72. DOI: http://dx.doi.org/10.1097/00001665-200311000-00008
9. Mitchell RN, Kumar V, Abbas AK. Fundamentos de Patologia: Robbins e Cotran. 8a ed. Rio de Janeiro: Elsevier; 2012.
10. Mathes SJ. Plastic Surgery: general principles. Philadelphia: Saunders Elsevier; 2006.
11. Pitanguy I. Utilização de expansores cutâneos nas sequelas de queimaduras. Bol Acad Nac Med. 1991;151(6/9):29-38.
12. Gur E, Hanna W, Andrighetti L, Semple JL. Light and electron microscopic evaluation of the pectoralis major muscle following tissue expansion for breast reconstruction. Plast Reconstr Surg. 1998;102(4):1046-51. PMID: 9734422 DOI: http://dx.doi.org/10.1097/00006534-199809020-00019
13. Jankovic J, Brin MF. Therapeutic uses of botulinum toxin. N Engl J Med. 1991;324(17):1186-94. PMID: 2011163 DOI: http://dx.doi.org/10.1056/NEJM199104253241707
1. Universidade Federal do Triângulo Mineiro,
Uberaba, MG, Brazil.
Corresponding author: Renata Margarida Etchebehere, Avenida Getúlio Guaritá, 130 - Uberaba, MG, Brazil. Zip Code 38025-440. E-mail: renataetch@hotmail.com
Article received: September 28, 2017.
Article accepted: September 5, 2018.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter