

Original Article - Year 2018 - Volume 33 -
Breast reconstruction with implants and synthetic mesh
Reconstrução mamária com implantes e tela sintética
ABSTRACT
Introduction: Mastectomy with immediate breast reconstruction
may prevent patients from experiencing a period of psychosocial
stress, negative body image, and sexual dissatisfaction. The
advent and implementation of novel materials such as implants,
expanders, and acellular dermal matrices have also contributed
to the success of breast reconstruction procedures. However,
the use of acellular dermal matrices in Brazil is restricted by
law and by their high cost. The objective of the present study
was to report the author's experience in breast reconstruction
with implants and synthetic mesh as an alternative to acellular
dermal matrices.
Method: This was a retrospective analysis of 12
consecutive patients (20 reconstructed breasts) who underwent
immediate or delayed breast reconstruction using the described
technique with implants and synthetic mesh between November
2015 and December 2016.
Results: Twelve patients (20 breasts)
were operated on using the technique described in this report.
The mean time of follow-up was 14 months. In this series, 15%
of patients had minor complications, including hematoma,
suture dehiscence, and rippling. The rate of complications was
similar to the rates reported in the literature, despite the limited
number of cases. The average degree of overall satisfaction with
the surgery was 75.2 points on a scale of 0-100, and the highest
score was given to breast appearance (85 points).
Conclusion:
Breast reconstruction with implants and synthetic mesh was
shown to be a technique with a low rate of complications,
high degree of patient satisfaction with the cosmetic result,
and decreased cost relative to acellular dermal matrices.
Keywords: Mammoplasty; Breast cancer; Breast implants; Simple mastectomy; Surgical meshes
RESUMO
Introdução: As mastectomias com reconstruções mamárias imediatas podem proteger a
paciente de um período de estresse psicossocial, imagem corporal negativa e
insatisfação sexual. O advento e utilização de novos materiais como os
implantes, expansores e matrizes dérmicas acelulares também contribuíram
para o sucesso das reconstruções mamárias. Porém, o uso das matrizes
dérmicas acelulares é restrito no Brasil pela legislação e seu alto custo. O
objetivo do estudo foi relatar a experiência do autor na reconstrução
mamária com implantes e tela sintética como uma alternativa às matrizes
dérmicas acelulares.
Método: Foi realizada uma análise retrospectiva de 12 pacientes consecutivas (20
mamas reconstruídas) que foram submetidas à reconstrução mamária imediata ou
tardia pela técnica descrita com implantes e tela sintética, entre novembro
de 2015 e dezembro de 2016.
Resultados: Doze pacientes (20 mamas) foram operadas pela técnica apresentada no estudo.
O tempo médio de follow-up foi de 14 meses. Nesta série,
15% apresentaram complicações menores como hematoma, deiscência de sutura e
rippling. O número de complicações, apesar do número
restrito de casos, é compatível com a literatura. O grau de satisfação
global com a cirurgia foi, em média, de 75,2 pontos em uma escala de 0-100,
sendo a nota mais alta atribuída à aparência das mamas (85 pontos).
Conclusão: A reconstrução mamária com implantes e tela sintética se mostrou uma técnica
com baixo índice de complicações, alto grau de satisfação das pacientes com
o resultado estético e com menores custos em relação ao uso de matrizes
dérmicas acelulares.
Palavras-chave: Mamoplastia; Neoplasias da mama; Implantes de mama; Mastectomia simples; Telas cirúrgicas
INTRODUCTION
Following the introduction of radical mastectomy by Halsted1, breast cancer surgery underwent a substantial evolution during the 20th century as a result of the incorporation of new techniques, new technologies, and the biomolecular analysis of tumors. Until the 1970s, the gold standard surgical treatment was radical mastectomy, which was considered a great success at the time.
However, the fear of mutilation and loss of quality of life felt by many women has motivated the search for less aggressive techniques for the locoregional control of the disease. Thus, interventions such as modified radical mastectomy, simple mastectomy, and conservative breast surgery have been included in the oncological surgery repertoire, with comparable results in terms of disease-free survival2 while offering the patients an improved perception of quality of life and body image3.
Advances in breast reconstruction surgery have occurred parallel to the evolution of ablative treatment of mastectomy patients. Surgery with autologous flaps advanced greatly with the use of microsurgery and the description of angiosomes4 and perforator-based flaps, which became excellent options for reconstruction, causing little or no damage to the donor area. The advent and implementation of novel materials developed in recent years, such as anatomical implants, expanders, and acellular dermal matrices, has also contributed to the success of breast reconstruction procedures.
Although breast reconstruction has obvious and significant benefits for the quality of life of mastectomy patients5, more than 60% of these women do not undergo the procedure6. The decision to have breast reconstruction involves a number of variables and may be associated with sociodemographic and ethnic factors or even medical conditions. Since 2013, a law in Brazil states that any woman undergoing mastectomy within the Unified Health System (SUS) should be guaranteed immediate reconstruction, in the context of favorable medical circumstances7.
Mastectomy with immediate breast reconstruction may prevent patients from experiencing a period of psychosocial stress, negative body image, and sexual dissatisfaction, as compared to late reconstruction8. Immediate breast reconstruction is typically performed using implants or expanders, especially in light of the increasing popularity of skin-sparing mastectomy, nipple-areola-complex (NAC)-sparing mastectomy, and even prophylactic mastectomy. It is estimated that reconstruction with implants will increase by an average of 5% per year9, owing to the acceptable rate of complications and proven oncological safety of this technique10.
The use of acellular dermal matrices has also contributed to the increased number of immediate implant-based reconstructions, as it provides better implant coverage, expansion of the submuscular pocket11, and reduced rates of capsular contracture12. However, most published studies have reported on the use of matrices of human origin. Since Brazilian laws do not permit the use of this type of product and the cost of dermal matrices of animal origin is still high in Brazil, new materials have been implemented with the aim of obtaining the obvious cosmetic benefits of these matrices.
The surgical use of synthetic meshes has been widely studied, and they have been shown to be safe, biocompatible, and hypoallergenic, with a low rate of complications13. Therefore, these materials may be effective replacements for dermal matrices in implant-based breast reconstruction surgery.
OBJECTIVE
This study aimed to report the author’s experience using a technique of implant-based breast reconstruction with synthetic mesh as an alternative to acellular dermal matrices.
METHOD
Patient selection
This was a retrospective analysis of 12 consecutive patients (20 reconstructed breasts) who underwent immediate or delayed breast reconstruction using the described technique with implants and synthetic mesh between November 2015 and December 2016.
All patients were operated by the author at the private clinic and at the breast center of the Integrated Oncology Center (Centro de Oncologia Integrado - COI) in Rio de Janeiro, RJ.
Decisions regarding patient selection, mastectomy technique indication, incision location, and possibility of immediate breast reconstruction with implants were made in consultation with the mastology team.
All patients underwent preoperative evaluation with detailed anamnesis, physical examination, laboratory testing, and X-rays. The following demographic data were obtained: age, history of the current disease, history of comorbidities, smoking habits, and previous neoadjuvant chemotherapy or radiotherapy. Preoperative photographic documentation was also included.
Physical examination included breast palpation and the measurement of breast width, height, and projection. Assessment of the contralateral breast (in cases of unilateral reconstruction), asymmetries, ptosis, thorax shape, and patient’s biotype was also performed. Together with the mastectomy technique, these data were essential in the estimation of the implant volume. All patients underwent breast reconstruction with textured anatomical implants Mentor® (Santa Barbara, CA) and semi-absorbable mesh ULTRAPRO® (Ethicon, a Johnson & Johnson company, Amersfoort, The Netherlands).
Surgical technique
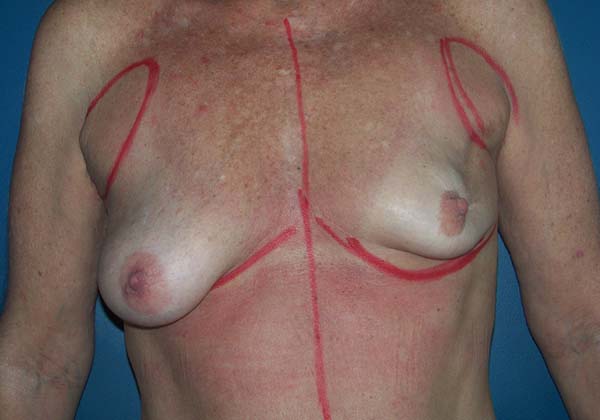
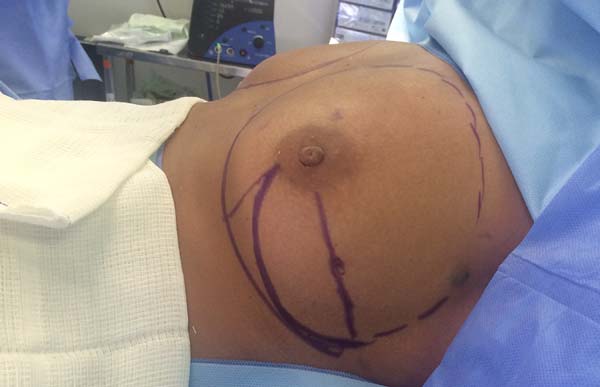
The location and type of incision are discussed with the mastology team, taking into account the tumor location, breast shape, ptosis, and patient’s expectations.
At the end of the mastectomy (skin-sparing, areola-sparing, or prophylactic), in cases of immediate reconstruction, the skin flap is assessed, as well as the patient’s oncological status. The flap is assessed clinically (color, capillary filling, and thickness). If there are signs of vascular injury, immediate reconstruction with implants is aborted and two-stage reconstruction with an expander is performed. In cases of the node-positive axilla, an indication for intraoperative adjuvant radiotherapy is another essential factor in determining the choice of technique. Due to the high incidence of capsular contracture in patients with implants who undergo radiotherapy, tissue expander-based reconstruction is another option.
Reconstruction begins with the detachment of the pectoralis major through its free margin and from the sternum and inframammary fold, without sectioning it and maintaining its attachment to the fascia. The size of the subpectoral pocket is calculated based on the desired volume of the implant and the patient’s anatomy. A mold with the desired volume of the implant is then inserted in the partial submuscular pocket to gauge the size of the fascia of the serratus anterior muscle that will be lifted to accommodate the lateral portion of the implant, where the mesh will be sutured.
After the dissection of the fascia of the serratus anterior muscle, the patient is seated on the operating table, the mold is once again inserted, and the incision is closed temporarily with a skin stapler. At this point, symmetry, contour, and position of the inframammary fold are assessed and the ideal implant is selected. The submuscular pocket is then irrigated with antibiotic solution (cefazolin + garamycin), and a vacuum suction drain and the implant are inserted.
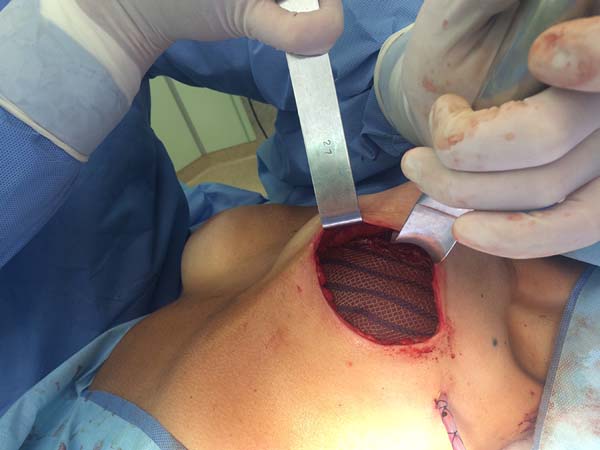
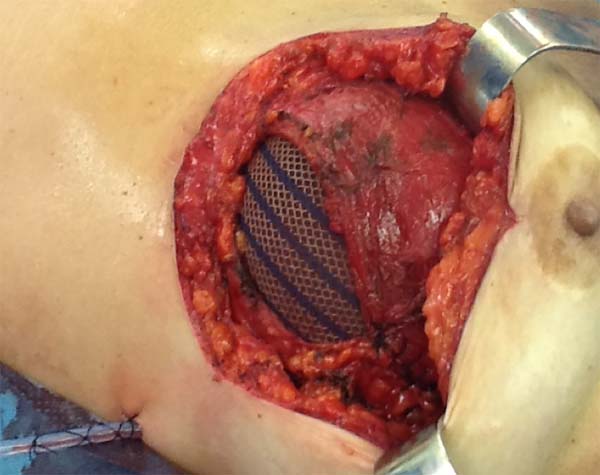
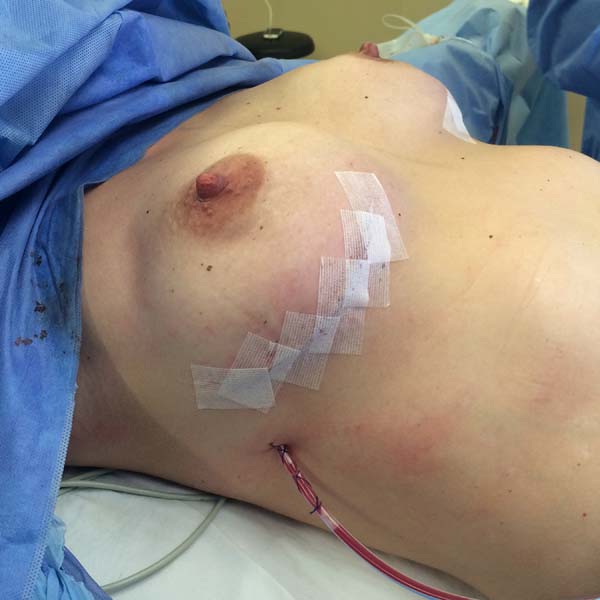
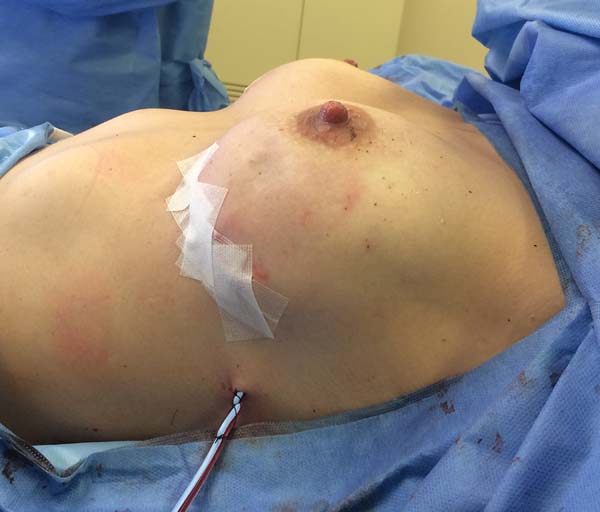
The superior-lateral portion of the pectoralis major is sutured to the fascia of the serratus anterior muscle with Vicryl 2-0, and the mesh is placed over the exposed portion of the implant. The mesh is then sutured with PDS thread 2-0 to the lateral margin of the pectoralis major and the fascia of the serratus anterior muscle to support the implant laterally and inferiorly (Figure 1). Intramuscular local anesthesia with bupivacaine solution (20 mL) is administered to reduce postoperative pain. The skin is then sutured in three layers, and the incision is covered with Steri-Strip® (Figures 2 and 3) and a surgical bra.
In one case of secondary reconstruction, the technique had to be slightly modified owing to insufficient muscle coverage. In that case, because the pectoralis major had been sectioned during the primary surgery, the lower lateral portion of the mesh was sutured to the serratus anterior muscle and the aponeurosis of the rectus abdominis muscle, while the upper portion was sutured to the small lobe of the remaining pectoralis major (Figure 4). In addition, capsulotomy or capsulectomy was performed in these cases.
Postoperative period
The patients were discharged from the hospital between 24 and 48 hours after surgery and administered antibiotics. Rest with moderate activity for a period of 30-45 days was recommended, along with the use of a surgical bra.
The patients were monitored weekly during the first postoperative month and then again at three and six months postoperatively. The drains were removed when drainage was less than 30 mL/day.
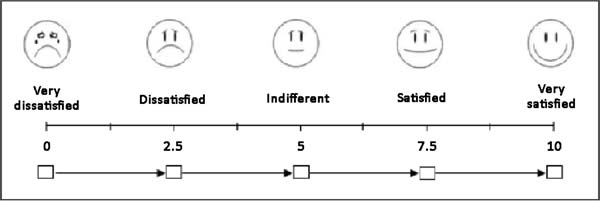
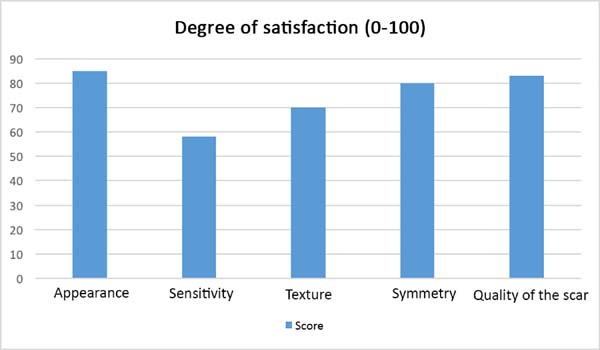
At postoperative 6 months, the patients were photographed and asked to describe their level of satisfaction regarding the surgical technique that was used by giving each parameter a score from 0 to 10, based on a visual analog scale (Figure 5). The following parameters were assessed: sensitivity, appearance, texture, symmetry, and scar quality. In addition, the patients were asked whether they would choose a different technique, with response options of “yes”, “maybe”, and “no”.
Lastly, the potential complications of the technique, including infection (requiring intravenous antibiotics), necrosis of the mastectomy flap or of the NAC, seroma, hematoma, suture dehiscence, rippling, and capsular contracture, were analyzed.
RESULTS
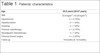
Twelve patients (20 breasts) underwent breast reconstruction with mixed mesh and implants between November 2015 and December 2016. The mean age of the patients was 55.6 years (35-67 years), and the mean body mass index (BMI) was 25.6 kg/m2 (19-29 kg/m2). The most common comorbidities were hypertension (16%) and hypothyroidism (8%). One patient (8%) was a smoker, two patients (16%) had a previous history of radiotherapy, one patient (8%) had a history of neoadjuvant chemotherapy, and three patients (25%) had undergone breast surgery. The mean follow-up time was 14 months (Table 1).
| Age | 55.6 years (36-67 years) |
|---|---|
| BMI | 25.6 kg/m2 (19-29 kg/m2). |
| Hypertension | n = 2 (16%) |
| Hypothyroidism | n = 1 (8%) |
| Smoking | n = 1 (8%) |
| Previous radiotherapy | n = 2 (16%) |
| Neoadjuvant chemotherapy | n = 1 (8%) |
| Previous breast surgery | n = 3 (25%) |
| Follow-up | 14 months (6-18 months) |
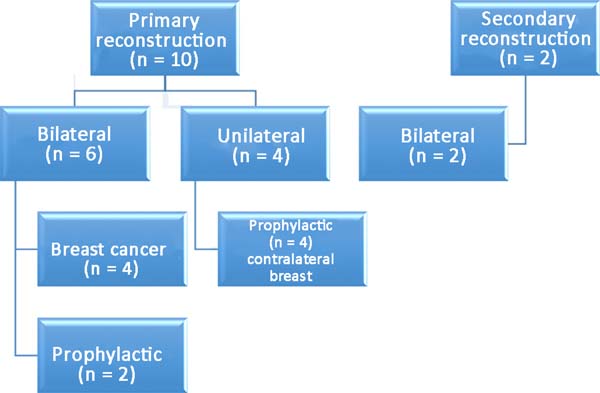
Among the patients, 83% (n=10) underwent primary breast reconstruction and 17% (n=2) underwent secondary breast reconstruction (Figure 6). The secondary reconstructions were performed in patients who had undergone conservative surgery for breast cancer with adjuvant radiotherapy and who exhibited severe asymmetry. Bilateral reconstructions accounted for 66% (n=8) of the cases, whereas unilateral reconstructions comprised 34% (n=4) of the cases. Considering only the bilateral reconstructions, 75% (n=6) were primary and 25% (n=2) were secondary reconstructions. Of the bilateral primary reconstructions, 66% (n=4) were NAC-sparing mastectomies for breast cancer and 34% (n=2) were prophylactic mastectomies in patients with the BRCA1 gene mutation.
In patients with the gene mutation, prophylactic adenomastectomy was associated with videolaparoscopic bilateral oophorectomy. The mean duration of unilateral surgeries was 100 minutes (80-120 min), while that of bilateral surgeries was 180 minutes (100-240 min). The mean volume of the used implants was 345 mL (270-415 mL). The mean duration of hospitalization was 36 hours (12-72 h). The mean time of drain use was 12.2 days.
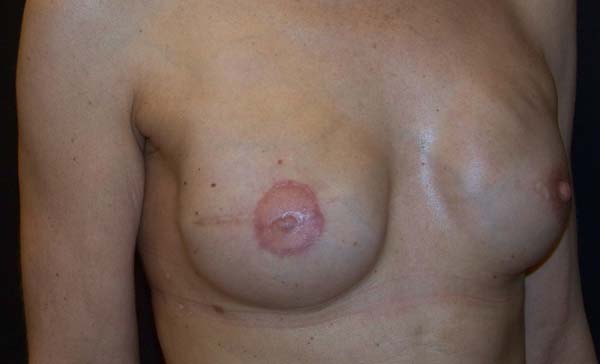
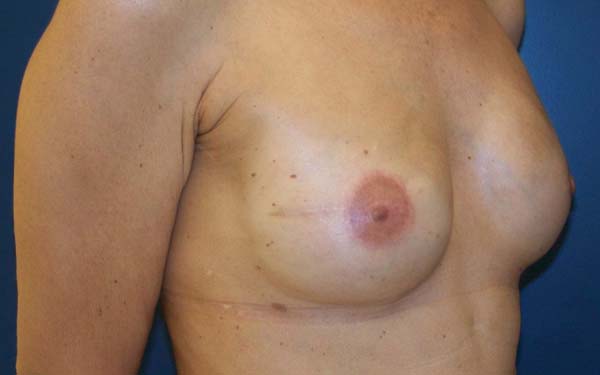
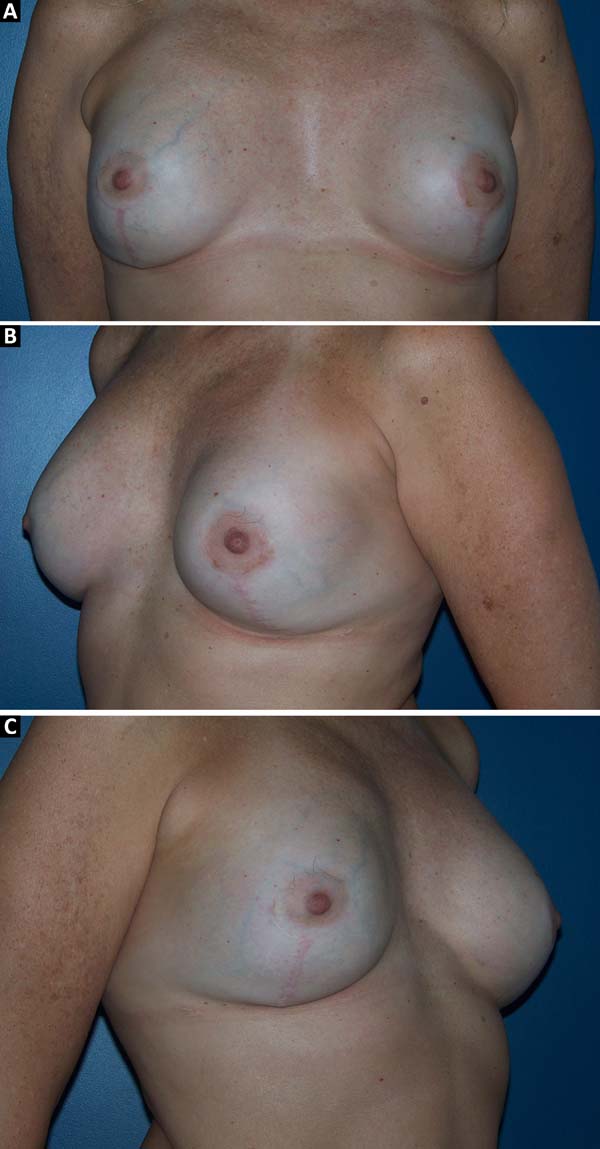
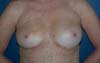
The complications are listed in Table 2. Three breasts (15%) had minor complications. One patient (5%) had a hematoma of moderate volume in the immediate postoperative period, which was treated conservatively with drainage in an outpatient setting. Another patient exhibited rippling at six months postoperatively, with spontaneous improvement after 12 months (Figures 7 and 8). Lastly, one patient had epidermolysis and suture dehiscence after unilateral adenomastectomy and breast reconstruction with implant and mesh. This patient was treated with surgical debridement and resuture because she resided in a different city and was not able to attend frequent follow-ups.
After the sixth month postoperatively, the patients answered the questionnaire regarding their degree of satisfaction. The mean score for satisfaction with breast appearance was 85 points. Sensitivity (58 points), texture (70 points), symmetry (80 points), and scar quality (83 points) were also scored. The overall mean was 75.2 points (Figure 9). Only one patient (6%) responded “maybe” when asked whether she would opt for a different surgical technique.
DISCUSSION
The benefits of immediate breast reconstruction have been proven with regard to improved quality of life in mastectomy patients, especially among young women14. Indeed, the level of psychosocial stress caused by the feeling of mutilation is alleviated in patients who undergo immediate reconstruction8.
The major advantage of immediate reconstruction with implants is that it is performed in a single stage, which means less patient morbidity and reduced costs. However, rigorous patient selection is mandatory to achieve a good cosmetic result after surgery. The decision to perform immediate reconstruction should be multifactorial and should consider important preoperative factors such as the patient’s oncological status, presence of comorbidities, smoking, and previous history of mammoplasty, neoadjuvant chemotherapy, or radiotherapy15.
Assessment of the mastectomy flap is another critical factor in the success of the surgery. This assessment may be performed clinically by analyzing patterns of ischemia or through studies of intraoperative imaging16. When intraoperative adjuvant radiotherapy is indicated, two-stage reconstruction with an expander should be selected, since irradiation of the implants is associated with increased complications17.
The success of the cosmetic result of immediate reconstructions with implants also hinges on the evolution of mastectomy surgical techniques, in particular skin-sparing and NAC-sparing mastectomies. The primary indications for this type of approach include prophylactic mastectomy or early-stage tumors; however, the spectrum of indications has been increasing. In the context of the appropriate indication, immediate reconstruction has been shown to be associated with a low rate of complications and good oncologic safety, even in patients with locally advanced tumors who underwent neoadjuvant chemotherapy18.
The use of acellular dermal matrices in breast reconstruction has allowed surgeons to obtain better cosmetic results as a result of less musculofascial dissection, better control of the inframammary fold with projection of the lower pole, and expansion of the submuscular pocket, which permits the safe use of larger implants19.
Despite these benefits, the use of acellular dermal matrices in Brazil is still restricted by the policies governed by Anvisa, which forbid the use of human (cadaveric) biological material in other patients. Dermal matrices of animal origin (porcine or bovine) are an alternative that yields similar results20, but their use is limited by their high cost. In general, it is estimated that the use of matrices is 8 to 10 times more expensive than that of meshes. Therefore, the use of a synthetic material that mimics biological matrices is being advocated with the aim of obtaining optimal aesthetic results.
In the present study, a partially absorbable, lightweight mesh made of synthetic materials, namely Prolene (polypropylene) and Monocryl (poliglecaprone), was used. The Monocryl portion is absorbed within 90-120 days and becomes incorporated into the tissue. The use of synthetic meshes in plastic surgery is not novel. In fact, many authors in Brazil have published studies on the subject, with a large number of citations pertaining to methods of mastopexy with mesh support21,22. The use of this synthetic material in breast reconstruction is also not new and has been shown to be a very safe technique with a low rate of complications, despite some technical differences between the published articles23,24.
The rigorous selection of appropriate patients is required for the success of the surgical technique. In the present study, 12 consecutive patients with a postoperative follow-up of at least six months were selected. Although obesity, smoking, and history of radiation may increase the risk of complications from this procedure15, a multivariate analysis of the patients’ characteristics as risk factors for complications was not performed owing to the small size of the sample. However, in the study cohort, the factors of the patients’ medical conditions, history of mammoplasty, and smoking appeared to have minimally impacted the rate of complications, with the viability of the mastectomy flap being the primary factor linked to the occurrence of epidermolysis and suture dehiscence.
The patients who underwent bilateral prophylactic adenomastectomy had a strong family history and BRCA1 gene mutation, and breast reconstruction in combination with videolaparoscopic bilateral oophorectomy was indicated. In addition, the association of an abdominal surgery did not appear to increase complications, even with an increased duration of surgery.
All patients who underwent unilateral procedures with this technique had undergone previous mastectomy and breast reconstruction with an expander or flap. During the exchange of the expander for the definitive implant or in a second stage, when indicated, these patients underwent contralateral prophylactic adenomastectomy and reconstruction with the technique in question. The patient who progressed to suture dehiscence and required reoperation was included in this group. In cases of prophylactic surgery, given the absence of local disease, it is essential to ensure that the mastectomy flap has an adequate thickness to avoid ischemic complications.
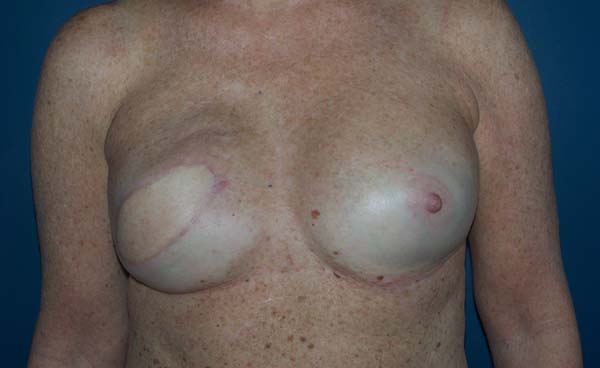
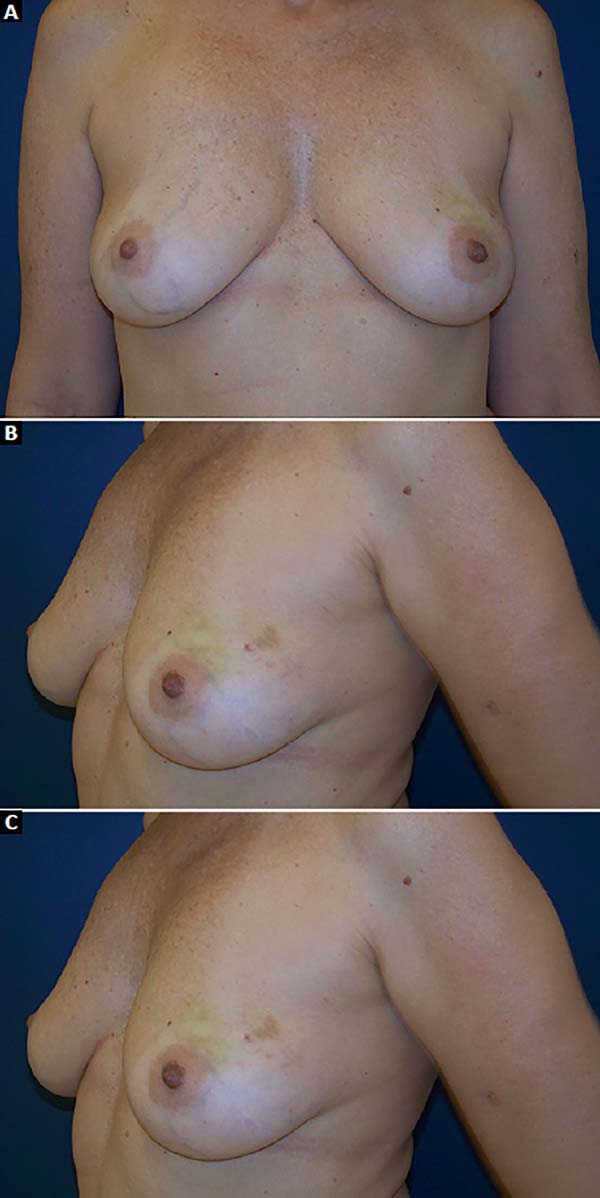
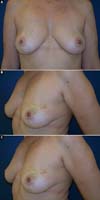
The patients who underwent secondary reconstruction exhibited the same profile, i.e., they had undergone conservative surgery and adjuvant radiotherapy and exhibited significant asymmetry. However, one patient had undergone reconstruction with implants at the time and progressed to capsular contracture in addition to asymmetry (Figures 10 to 13).
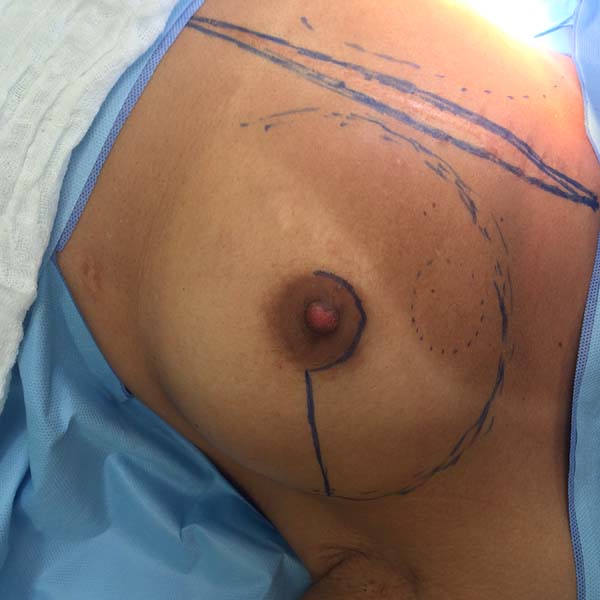
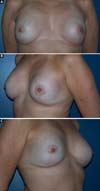
Another factor that is essential to reduce complications is the location of the incisions. This should be discussed and planned based on the mastologist’s experience, tumor location (in the case of cancer), and presence or absence of previous scars. In the present study, there were incisions along the submammary fold, comma-shaped inferior incisions (Figure 14), periareolar incisions with lateral extension (Figure 15), and classical mammoplasty incisions.
Incisions that involve the areola appear to be more associated with ischemic complications of the NAC25. Thus, patients at higher risk of NAC necrosis after mastectomy, such as smokers or patients with previous mammoplasty, may undergo areola autonomization in an outpatient setting26 to improve local vascularization. However, no patient in the present study underwent this procedure. It is very important that the mastectomy flap is well vascularized, especially when incisions are made to elevate the NAC.
One of the main complaints of patients in the postoperative period is pain, which is primarily caused by the major musculofascial dissection. Therefore, the aim of using intramuscular bupivacaine was to control pain and reduce the need for opioids postoperatively. Although the results of using liposomal bupivacaine are promising27, its use is still not permitted in Brazil. Recent studies analyzing immediate breast reconstruction with implants covered by dermal matrices in the prepectoral position also aimed to reduce postoperative pain as well as complications such as hyper-animation deformity, but they remain very controversial28.
Despite the small size of the study sample, the rate of complications of reconstruction surgery with implants and mesh was similar to that observed in other large series29,30. The degree of satisfaction of the patients was measured using a visual analog scale comprising 5 parameters. While the best method for assessing the patients’ degree of satisfaction in terms of quality of life after breast reconstruction is a validated and translated questionnaire, such as the Breast-Q31, it is a long questionnaire that patients have difficulty completing in the private clinic setting. Therefore, it was not used in the present study.
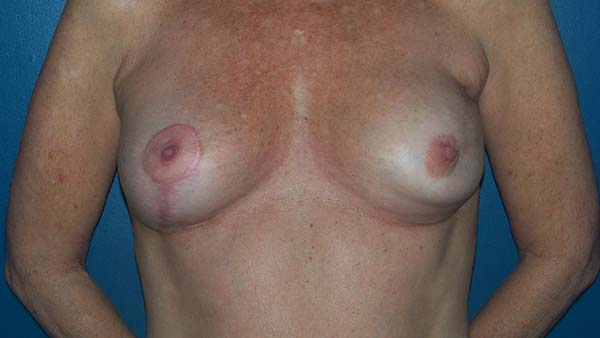
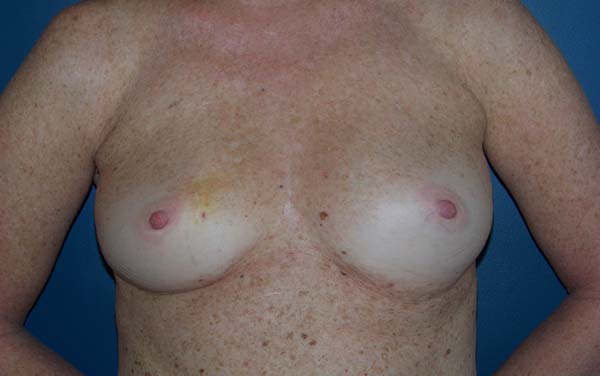
The patients were primarily dissatisfied with the sensitivity; however, because the questionnaire was administered at six months postoperatively, these patients would likely report some degree of improvement in a later follow-up. Although the parameter “texture” had a good satisfaction score in the questionnaire, some patients reported feeling the surface of the mesh, especially in thin flaps. However, this complaint was not frequent after the period of absorption of the PDS thread, which is used in the fixation of the mesh to the muscle wall, and of the Monocryl portion of the mesh. The patients’ degree of satisfaction with the cosmetic result also scored well, which validates the continued application of the technique (Figures 16 to 19).
Although this was a retrospective study with a small number of cases, the results demonstrated that the proposed reconstruction technique with implants and mixed mesh has a lower rate of complications compared to other techniques of immediate reconstruction with implants with total muscle coverage or the use of dermal matrices.
Studies on the placement of implants with total muscle coverage indicate complication rates of up to 40%, including mainly implant malposition, asymmetry of the submammary fold, and capsular contracture30. Therefore, the limitations of this technique are based on the small size of the submuscular pocket, which prevents the placement of larger implants and hinders the creation of a natural breast and a defined submammary fold. The use of a mesh helps to enlarge the pocket and allows for better control of the implant positioning, as well as greater expansion of the lower pole of the breast.
A prospective, randomized, and controlled study is necessary to compare the use of mesh with that of acellular dermal matrices, including an assessment of cost-effectiveness. Matrix-related complications are usually associated with infection and seroma, with rates ranging between 6% and 29%32,33. In the present study, the complications were minor and occurred at a rate of 15%.
CONCLUSION
The proposed breast reconstruction technique using implants and synthetic mesh was found to be associated with a low rate of complications, a high degree of patient satisfaction with the cosmetic result, and a lower cost relative to the use of acellular dermal matrices. However, rigorous patient selection, careful incision planning, and a well-vascularized flap after mastectomy are extremely critical factors for the success of the surgical procedure.
REFERENCES
1. Halsted WS. I. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg. 1907;46(1):1-19. PMID: 17861990
2. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233-41. PMID: 12393820 DOI: http://dx.doi.org/10.1056/NEJMoa022152
3. Al-Ghazal SK, Fallowfield L, Blamey RW. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer. 2000;36(15):1938-43. DOI: http://dx.doi.org/10.1016/S0959-8049(00)00197-0
4. Taylor GI. The angiosomes of the body and their supply to perforator flaps. Clin Plast Surg. 2003;30(3):331-42. DOI: http://dx.doi.org/10.1016/S0094-1298(03)00034-8
5. Reuben BC, Manwaring J, Neumayer LA. Recent trends and predictors in immediate breast reconstruction after mastectomy in the United States. Am J Surg. 2009;198(2):237-43. DOI: http://dx.doi.org/10.1016/j.amjsurg.2008.11.034
6. Alderman AK, Wei Y, Birkmeyer JD. Use of breast reconstruction after mastectomy following the Women's Health and Cancer Rights Act. JAMA. 2006;295(4):387-8. PMID: 16434628
7. Brasil. Governo Federal. Ministério da Saúde. Lei Nº 9.797, de 6 de maio de 1999. Alterada pela Lei Federal 12.802, de 24/04/2013. Dispõe sobre a obrigatoriedade da cirurgia plástica reparadora da mama pela rede de unidades integrantes do Sistema Único de Saúde - SUS nos casos de mutilação decorrentes de tratamento de câncer. Brasília: Governo Federal; 2013.
8. Zhong T, Hu J, Bagher S, Vo A, O'Neill AC, Butler K, et al. A Comparison of Psychological Response, Body Image, Sexuality, and Quality of Life between Immediate and Delayed Autologous Tissue Breast Reconstruction: A Prospective Long-Term Outcome Study. Plast Reconstr Surg. 2016;138(4):772-80. PMID: 27673514 DOI: http://dx.doi.org/10.1097/PRS.0000000000002536
9. Albornoz CR, Bach PB, Mehrara BJ, Disa JJ, Pusic AL, McCarthy CM, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15-23. PMID: 23271515 DOI: http://dx.doi.org/10.1097/PRS.0b013e3182729cde
10. Munhoz AM, Aldrighi CM, Montag E, Arruda EG, Aldrighi JM, Gemperli R, et al. Clinical outcomes following nipple-areola-sparing mastectomy with immediate implant-based breast reconstruction: a 12-year experience with an analysis of patient and breast-related factors for complications. Breast Cancer Res Treat. 2013;140(3):545-55. PMID: 23897416 DOI: http://dx.doi.org/10.1007/s10549-013-2634-7
11. Macadam SA, Lennox PA. Acellular dermal matrices: Use in reconstructive and aesthetic breast surgery. Can J Plast Surg. 2012;20(2):75-89. DOI: http://dx.doi.org/10.1177/229255031202000201
12. Maxwell GP, Gabriel A. Use of the acellular dermal matrix in revisionary aesthetic breast surgery. Aesthet Surg J. 2009;29(6):485-93. DOI: http://dx.doi.org/10.1016/j.asj.2009.09.007
13. Shulman AG, Amid PK, Lichtenstein IL. The safety of mesh repair for primary inguinal hernias: results of 3,019 operations from five diverse surgical sources. Am Surg. 1992;58(4):255-7. PMID: 1586085
14. Dauplat J, Kwiatkowski F, Rouanet P, Delay E, Clough K, Verhaeghe JL, et al.; STIC-RMI working group. Quality of life after mastectomy with or without immediate breast reconstruction. Br J Surg. 2017;104(9):1197-206. DOI: http://dx.doi.org/10.1002/bjs.10537
15. Tang R, Coopey SB, Colwell AS, Specht MC, Gadd MA, Kansal K, et al. Nipple-Sparing Mastectomy in Irradiated Breasts: Selecting Patients to Minimize Complications. Ann Surg Oncol. 2015;22(10):3331-7. DOI: http://dx.doi.org/10.1245/s10434-015-4669-y
16. Gurtner GC, Jones GE, Neligan PC, Newman MI, Phillips BT, Sacks JM, et al. Intraoperative laser angiography using the SPY system: review of the literature and recommendations for use. Ann Surg Innov Res. 2013;7(1):1. DOI: http://dx.doi.org/10.1186/1750-1164-7-1
17. Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124(2):395-408. PMID: 19644254 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181aee987
18. Coopey SB, Tang R, Lei L, Freer PE, Kansal K, Colwell AS, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol. 2013;20(10):3218-22. DOI: http://dx.doi.org/10.1245/s10434-013-3152-x
19. Vardanian AJ, Clayton JL, Roostaeian J, Shirvanian V, Da Lio A, Lipa JE, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128(5):403e-410e. PMID: 22030500
20. Glasberg SB, Light D. AlloDerm and Strattice in breast reconstruction: a comparison and techniques for optimizing outcomes. Plast Reconstr Surg. 2012;129(6):1223-33. PMID: 22327891 DOI: http://dx.doi.org/10.1097/PRS.0b013e31824ec429
21. Sampaio Góes JC. Periareolar mammaplasty: double-skin technique with application of mesh support. Clin Plast Surg. 2002;29(3):349-64. DOI: http://dx.doi.org/10.1016/S0094-1298(02)00005-6
22. Bozola AR. Mamoplastia pós-cirurgia bariátrica usando suporte protético complementar de contenção glandular. Rev Bras Cir Plást. 2016;31(3):299-307.
23. Tessler O, Reish RG, Maman DY, Smith BL, Austen WG Jr. Beyond biologics: absorbable mesh as a low-cost, low-complication sling for implant-based breast reconstruction. Plast Reconstr Surg. 2014;133(2):90e-9e. PMID: 24469217 DOI: http://dx.doi.org/10.1097/01.prs.0000437253.55457.63
24. Pukancsik D, Kelemen P, Gulyás G, Újhelyi M, Kovács E, Éles K, et al. Clinical experiences with the use of ULTRAPRO® mesh in single-stage direct-to-implant immediate postmastectomy breast reconstruction in 102 patients: A retrospective cohort study. Eur J Surg Oncol. 2017;43(7):1244-51. DOI: http://dx.doi.org/10.1016/j.ejso.2017.01.236
25. Rawlani V, Fiuk J, Johnson SA, Buck DW 2nd, Hirsch E, Hansen N, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can J Plast Surg. 2011;19(4):129-33. DOI: http://dx.doi.org/10.1177/229255031101900410
26. Jensen JA, Lin JH, Kapoor N, Giuliano AE. Surgical delay of the nipple-areolar complex: a powerful technique to maximize nipple viability following nipple-sparing mastectomy. Ann Surg Oncol. 2012;19(10):3171-6. DOI: http://dx.doi.org/10.1245/s10434-012-2528-7
27. Leiman D, Barlow M, Carpin K, Piña EM, Casso D, et al. Medial and lateral pectoral nerve block with liposomal bupivacaine for the management of postsurgical pain after submuscular breast augmentation. Plast Reconstr Surg Glob Open. 2015;2(12):e282.
28. Sigalove S, Maxwell GP, Sigalove NM, Storm-Dickerson TL, Pope N, Rice J, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast Reconstr Surg. 2017;139(2):287-94. DOI: http://dx.doi.org/10.1097/PRS.0000000000002950
29. Baldelli I, Cardoni G, Franchelli S, Fregatti P, Friedman D, Pesce M, et al. Implant-Based Breast Reconstruction Using a Polyester Mesh (Surgimesh-PET): A Retrospective Single-Center Study. Plast Reconstr Surg. 2016;137(6):931e-9e. PMID: 27219260 DOI: http://dx.doi.org/10.1097/PRS.0000000000002180
30. Meyer Ganz O, Tobalem M, Perneger T, Lam T, Modarressi A, Elias B, et al. Risks and benefits of using an absorbable mesh in one-stage immediate breast reconstruction: a comparative study. Plast Reconstr Surg. 2015;135(3):498e-507e. DOI: http://dx.doi.org/10.1097/PRS.0000000000001027
31. Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345-53. PMID: 19644246 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181aee807
32. Chun YS, Verma K, Rosen H, Lipsitz S, Morris D, Kenney P, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125(2):429-36. PMID: 20124828 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181c82d90
33. Lanier ST, Wang ED, Chen JJ, Arora BP, Katz SM, Gelfand MA, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64(5):674-8. PMID: 20395795
1. Sociedade Brasileira de Cirurgia Plástica, São
Paulo, SP, Brazil.
2. Américas Centro de Oncologia Integrado, Rio de
Janeiro, RJ, Brazil.
3. Hospital Federal de Ipanema, Rio de Janeiro,
RJ, Brazil.
*Corresponding author: Daniel Gouvea Leal, Rua Redentor, 26 - Rio de Janeiro, RJ, Brazil. Zip Code 22421-030. E-mail: danileal.rlk@terra.com.br
Article received: June 5, 2017.
Article accepted: August 7, 2017.
Conflicts of interest: none.













 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter