

Articles - Year 1999 - Volume 14 -
Subfascial Endoscopic Transaxillary Augmentation Mammaplasty
Mamaplastia de Aumento Transaxilar Videoendoscópica Subfascial
ABSTRACT
Videoendoscopy for breast hypoplasia and glabelar frownlines has been used since 1996 in our private clinic. Breast augmentation with "S" shape incision for transaxillary acessis utilized to introduce the implant, in a submuscular or subglandular, and recently (since october 1998) in a subfascial location. From August 1998 through January 1999, 62 patients underwent endoscopics surgeries, forty nine were submuscular, five subglandular and eight subfascial. McGhan® 410, anatomical biodimentional, 155 grams through 235 grams were used. We observed three cases of complications, two of them malpositioning (rotation) needing reoperation and 1 hematoma treated with drainage. Patient satisfaction was high, especially regarding the axillary incision. There have been no capsular contractions to date.
Keywords: Mastoplasty; endoscopy; mammary surgery.
RESUMO
No nosso serviço, a prática da cirurgia videoendoscópica começou em 1996, sendo utilizada para tratamento de hipoplasia mamária e de rugas frontais, que seriam as cirurgias videoendoscópicas mamária e facial. Na mamaplastia de aumento foi utilizada a via transaxilar com incisão em forma de "S" para introduzir o implante mamário na posição submuscular ou subglandular e, mais recentemente (outubro de 1998), na posição subfascial. Essa cirurgia foi realizada em 62 pacientes, no período de agosto de 1996 a janeiro de 1999, sendo introduzidas as próteses no espaço submuscular em 49 pacientes, subglandular em 5 pacientes e subfascial em 8 pacientes. O tipo de implante utilizado foi o modelo anatômico 410, biodimensional da McGhan®, com variação de 155 a 235 gramas. Ocorreram complicações em três pacientes, sendo que houve a rotação da prótese com assimetria em duas delas com necessidade de reoperação e, em uma paciente, formou-se um hematoma, que foi resolvido por punção. O grau de satisfação pessoal foi alto, sendo a maior vantagem dessa técnica a ausência de cicatriz nas mamas. Nenhum dos casos até o momento apresentou algum grau de contratura capsular.
Palavras-chave: Mastoplastia; endoscopia; cirurgia mamária.
Transaxillary breast augmentation has presented many advantages over other techniques(5, 7, 12). The mainstay is the absence of scar on the breast.
The rationale for place the implant submuscularly, and recently subfascially, is to reduce the capsular contraction incidence in the late post-operative period and to avoid areolar sensation disturbances(1, 9, 10, 13, 15).
The use of endoscopic magnifiing lenses and video amplifies the images and gives the surgeon a better visualization of tissues and planes allowing more precise dissection and hemostasis while using only a small axillary incision(3, 6, 9).This technique is not indicated for moderate and severe ptosis.
Breast endoscopic surgery was first described and used since 1987, for internal capsulotomy and to evaluate mammary implants(2, 4, 8).
Johnson and Christ (1993) first described the videoendoscopic approach in transumbelical breast augmentation and in the same year Laurence Ho published his experience with transaxillary endoscopic augmentation(6). In 1994, Price, Nahai and Bostwick published the endoscopic transaxillary subpectoral breast augmentation with good aesthetic results and no complications(11).
MATERIAL AND METHODS
Sixty two patients underwent transaxillary endoscopic breast augmentation, from August 1996 through January 1999. 49 were located in the subpectoral plane, five were subglandular and eight were subfascial.
The age varied from 15 to 48 years old. The textured, biodimentional, high cohesiviness silicon gel, MeGhan® 410 implants, sizing 155 through 235 grams was used.
The inframammary sulcus was demarcated with the patient in the upright position and two em below the neosulcus line, parallel to the original sulcus,another line was placed. The area to be undermined is delineated.
These procedures were done under general anesthesia and the arms abducted to 90° with slight eleavtion of the dorsum. The incision was "S"shape marked in the axillary cavum, 3 cm long and one cm in the posterior portion of the major pectoralis muscle border. This allows direct access to the rectropectoral or prepectoral (subglandular) or subfascial plane. The dissection was performed utilizing videoendoscopy electrocautery and high frequency cautery, endoscopic scisors, hemostats, dissectors and endoretractors.
When subglandular access was utilized(3), undermining was performed one cm bellow the original submammary sulcus and superiorly to the second intercostal space. When submuscular access(13, 16) was chosen, the inferior and inferomedial insertion of the pectoralis muscle to the sternum and ribs was sectioned, respecting 1 cm of its osteous insertion to facilitate eventual bleeders hemostasis. This undermining was performed until 2 cm below the original submammary sulcus, because muscular contraction may bring the implant upward. When using subfascial access (the prefered approach since October 1998), the dissection has to begin in the lateral border of the pectoralis muscle, accessing the subfascia with gentle movements to proceed the undermining upward to the second intercostal space and inferiorly to the level of the 5th and 6th intercostal space, where the junction of the pectoralis fascia and abdominus rectus and lateral oblique muscle is found. At this point the fascia is tender but resistant and from this point inferiorly, undermining shifts to a suprafascial or subglandular plane until it reaches one or two cm bellow the original submammary sulcus. Once undermining is completed and with the hemostasis under direct view, the implant is inserted. The implant is marked with methylene blue on its superolateral quadrant, which can be seen under endoscopy in order to avoid rotation.
Closed drainage is mantained for 24 hours. Dressing with an elastic band in the upper thorax to mantain the implant in the correct position, avoiding upward dislocation, is used for 30 days. Physiotherapy on the breast begins in the 7th postoperative day, allowing the patient to return to its regular activities after this date.
RESULTS
Complications are infrequent. Out of sixty two patients operated during a three years follow-up, only three complications were observed. They consisted of one case with hematoma, which was drained during ultrassonographic assistance and two patients with implants malpositioning which were reoperated through the same access, endoscopically, repositioning the implant (Figs. 1 and 3). There was one patient with axillary muscle contraction that resolved with physiotherapy. Ecchymosis and edema subside in a few weeks and patients returned to activities in 7 days.
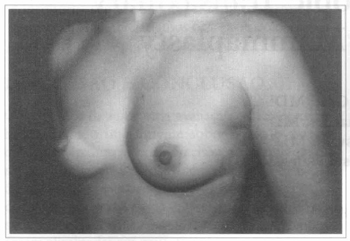
Fig. 1 - A thirty one years old female patient, with hypoplastic breasts.
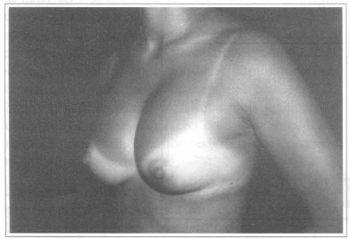
Fig. 2 - Same patient, six months after transaxillary videoendoscopic breast implant with asymmetry of the superior part of the left breast.
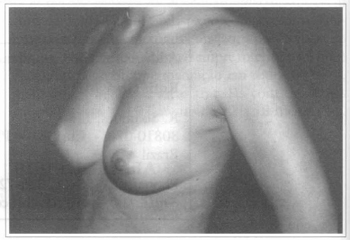
Fig. 3 - Same patient six months after transaxillary videoendoscopic breast implant reoperation to improve implant's rotation.
Subfascial implants offer better contouring of the breast with a more natural appearance.
Personal satisfaction in all cases was excelent. The reasons for this relies on the absence of breast scars combined with almost imperceptible axillar scars and a much better shape of the breast at late postoperative period (Figs. 4 and 5).
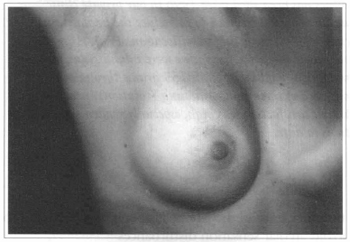
Fig. 4 - Axillary scar four months after videoendoscopic surgery.
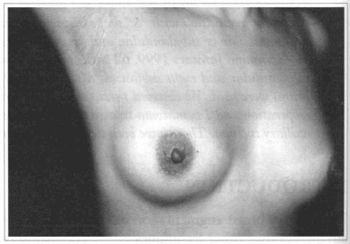
Fig. 5 - Same patient one year after surgery.
COMMENTS
The submuscular space, which minimizes capsular contracture has been our choice for 17 years (Figs. 6 and 7). The muscular movements during activities maintain a constant massage to the implant and give a more natural look and texture to the breast.
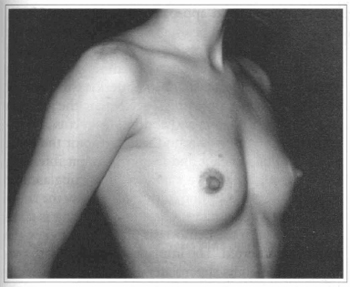
Fig. 6 - A 26 years old female patient with hypoplasic breast.

Fig. 7 - Same patient six months after transaxillary videoendoscopic submuscular mammaplasty
Neverthless there was a 3% capsular contracture rate.
Since four year ago we began using high cohesiviness breast implants and there was a striking reduction of contracture to zero. In one patient who was reoperated in the 6th postoperative month for repositioning of the implant, the capsule was sent for analysis and no silicone infiltration or leakeage was revealed. This suggests that silicone leakage may be a reason for capsular contracture.
Based on these data and by observing that in some patients with submuscular implants the inferior pole of the breast was flattened during physical activity, we began to use the subfascial implant. In the area from the 2nd to the 5th and 6th intercostal space the undermining is carried just above the muscle fibers and just bellow the pectoral fascia. Inferiorly to these point the undermining moves to subglandular untill one to two em bellow the submammary sulcus. The subfascial implant has given a more natural look to the breast, avoiding flattening or change in shape of the breast as it occours with submuscular implants. Another point is that the implant's edge is not marked on the breast as it may occour with the subglandular location (Figs. 8 and 9), as in severe breast hypoplasia. This last option is maintained for those patients with enough breast tissue to hide the implants borders.
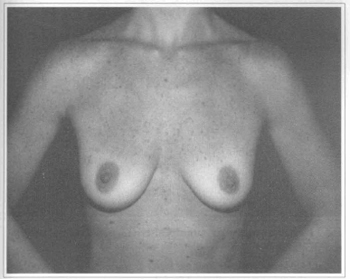
Fig. 8 - A 29 years old female patient with flaccidity and hypoplasic breast.
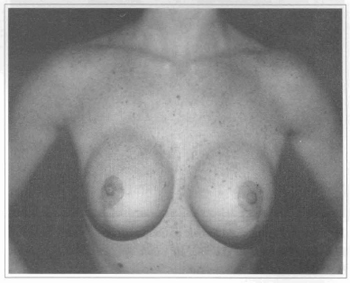
Fig. 9 - Same patient eight months after mastopexy and subglandular breast implant with visible borders at the superior part of the breast.
CONCLUSION
The videoendoscopic transaxillary mammaplasty seems to be a safe alternative to breast augmentation and gives better and more natural result, improving patients satisfaction, as far as scar, shape and low complication rate are concerned. Recently, subfascial access has been our choice for breast augmentation. The main reasons for this are that at the upper pole the implant looks like a submuscular implant without sharp demarcations while at the lower pole the breast looks like a subglandular implant without shape flattening as it occurs in submuscular implant. The final result is a more natural breast shape with the subfascial implant (Figs. l0 to 20).
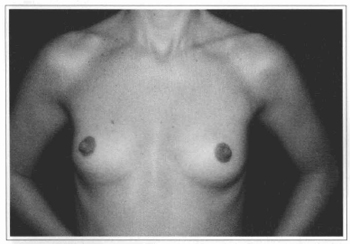
Fig.l0 - A 31 years old female patient with hypoplastic breast.
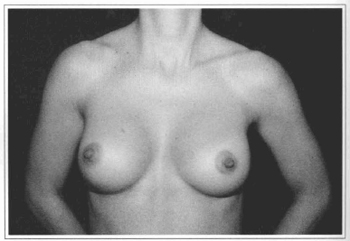
Fig 11 - Same patient six months after videoendoscopic sub-fascial mammaplasty.
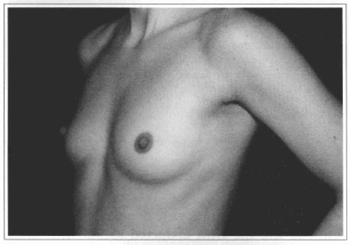
Fig. 12- Same patient of Fig. 10, oblique view, before surgery.
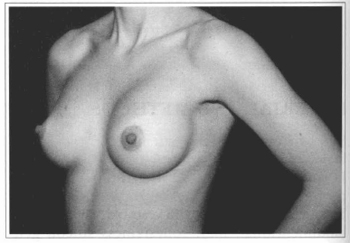
Fig. 13- Same patient of Fig. 10, oblique view, six months after surgery.
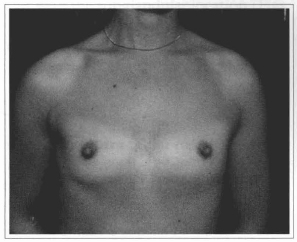
Fig. 14 - A 27 years old female patient with hypoplasic breast.
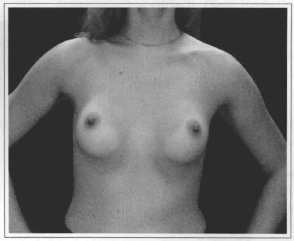
Fig. 15- Same patient six months after videoendoscopic sub-fascial mammaplasty.
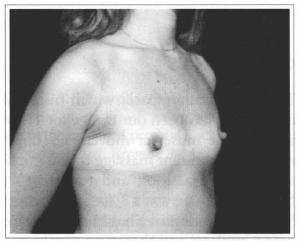
Fig. 16- Same patient of Fig. 14, oblique view, before surgery.
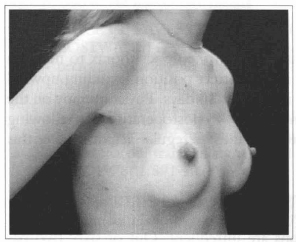
Fig. 17- Same patient of Fig. 14, oblique view, six months after surgery.
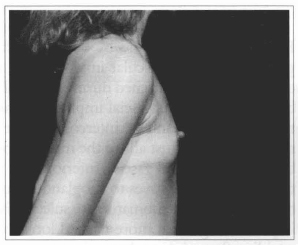
Fig. 18- Same patient of Fig. 14, profile view, before surgery.
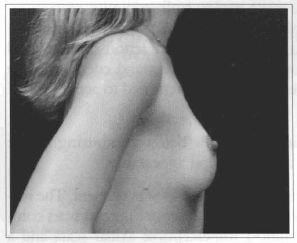
Fig. 19- Same patient of Fig. 14, profile view, six months after surgery.
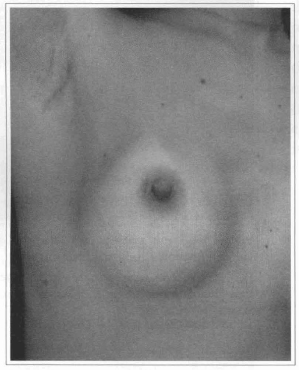
Fig. 20- Axillary scar six months after videoendoscopic transaxillary breast augmentation without scar at the mammary unit.
REFERENCES
1. BARNETT A. Transaxillary subpectoral augmentation in the ptotic breast: augmentation by disruption of the extended pectoral fascia and parenchymal sweep. Plast. Reconstr Surg. 1990; 86:76-83.
2. BEER GM, KOMPATSCHER P. Endoscopic plastic surgery: the endoscopic evaluation of implants after breast augmentation. Aest. Plast. Surg. 1995; 19:353-359.
3. CHACHIR A, BENZAQUEN I, SPAGNOLO N, LUSICIC N. Endoscopic augmentation mastoplasty Aesthet. Plast. Surg. 1994; 18:377-382.
4. DOWDEN RV,ANAIN S. Endoscopic implant evaluation and capsulotomy. Plast. Reconstr Surg. 1993; 91:283-287.
5. EISEMAN G. Augmentation mammaplasty by the transaxillary approach. Plast. Reconstr Surg. 1974; 54:229-232.
6. HO LCY Endoscopic assisted transaxillary augmentation mammaplasty Br. J. Plast. Surg. 1993; 46: 332-336.
7. HOEHLER H. Breast augmentation: the axillary approach. Br. J. Plast. Surg. 1973; 26:373-376.
8. HÖHLER H. Further progress in the axillary approach in augmentation mammaplasty: prevention of incapsulation. Aesthet. Plast. Surg. 1977; 1:107- 113.
9. HOWARD PS, OSLIN BD, MOORE JR. Endoscopic transaxillary submuscular augmentation mammaplasty with textured saline breast implants. Annals Plast. Surg. 1996; 37(1):12-17.
10. PAPILLON J. Pros and cons of subpectoral implantation. Clin. Plast. Surg. 1976; 3(2):321-337.
11. PRICE CI, EAVES FE.3rd, NAHAI F, JONES G, BOSTWICK J 3rd. Endoscopic transaxillary subpectoral breast augmentation. Plast. Reconstr Surg. 1994; 94:612-619.
12. RAYNOR AC, KLEIN AW,HABAL MB. Cosmetic advantage of augmentation the hypoplastic breast via the transaxillary route. Aesthet. Plast. Surg. 1978; 1:391-407.
13. REGNAULT P. Partially submuscular breast augmentation. Plast. Reconstr. Surg. 1977; 59:72-76.
14. SASAKI GH. Endoscopically assisted transaxillary augmentation mammaplasty Endosc. Aesthet. Reconstr Surg. 1996; 7:105-119.
15. TEBBETTS JB. Transaxillary subpectoral augmentation mammaplasty: long-term follow-up and refinements. Plast. Reconstr. Surg. 1984; 74:636-649.
16. WRIGHT JH, BEVIN G. Augmentation mammaplasty by the transaxillary approach. Plast. Reconstr Surg. 1976; 58:429-433.
I - Senior Member of the Brazilian Society of Plastic Surgery, Member of the ISAPS, President of the Scientific Cornrnittee of the Brazilian Society of Plastic Surgery, Section of Paraná, Guest Professor of the Evangelic University of Curitiba.
II - Especialist Member of the Brazilian Society of Plastic Surgery.
Address for correspondence:
Ruth Graf, MD
R. Solimões, 1184
80810-070 - Curitiba - PR Brazil
Phone: (55-41) 335-7237 Fax: (55-41) 335-9394
e-mail: hansgraf@bsi.com.br


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter