

Original Article - Year 2018 - Volume 33 -
Breast reconstruction with creation of upper retropectoral and lower mixed subcutaneous cavities and use of flaps
Reconstrução mamária com loja de retalho retropeitoral superior e subcutâneo misto inferior
ABSTRACT
Introduction: A total of 57,960 new cases of breast cancer were expected in Brazil in 2016
according to data from the National Cancer Institute, corresponding to 25%
of cancers in the country (excluding non-melanoma skin tumors). This study
aims at presenting a surgical approach for immediate breast reconstructions
with upper retropectoral and lower mixed subcutaneous cavities.
Methods: The authors performed breast reconstruction using flaps of the pectoral
muscle and inferior cutaneous pedicle associated with insertion of silicone
breast implants. The medical records of patients operated between 2012 and
2016 at the Plastic Surgery Service of the senior author's private clinic at
Daher Hospital and Armed Forces Hospital were retrospectively analyzed.
Results: The results were satisfactory, with low complication rates and high patient
and author satisfaction. Thirty-six patients, with a mean age of 59 years,
underwent breast reconstruction using the described technique. The
complications were nipple-areola complex necrosis, dehiscence, seroma,
hematoma, liponecrosis, implant displacement, and deep venous thrombosis. No
patient needed salvage surgery or had recurrence of mammary neoplasia during
the study period.
Conclusion: The present technique preserves the skin located at the lower portion of the
breast, with a low risk of dehiscence or extrusion of the implant in this
region, providing a double protection of the implant, with the pectoralis
major muscle in the upper two thirds and the cutaneous-dermis-fat flap in
the lower third, characterizing a "dual-plane" positioning.
Keywords: Mastectomy; Breast neoplasms; Mammoplasty
RESUMO
Introdução: Segundo dados do Instituto Nacional de Câncer (INCA) de 2016, estima-se em
57.960 novos casos de câncer de mama no Brasil, o que corresponde a 25%
dentre todos os tipos de cânceres no país (excluindo-se os tumores de pele
não melanoma). O presente artigo visa apresentar uma forma de abordagem para
as reconstruções mamárias imediatas com loja retropeitoral superior e
subcutânea mista inferior.
Métodos: Os autores descrevem da técnica de reconstrução mamária com retalho do
músculo peitoral e pedículo cutâneo inferior, associado à inclusão de
implante mamário de silicone. Foi realizada análise retrospectiva de
prontuários das pacientes operadas entre os anos 2012 e 2016 no Serviço de
Cirurgia Plástica da clínica privada do autor sênior, no Hospital Daher e no
Hospital das Forças Armadas.
Resultados: Os resultados são satisfatórios, com baixos índices de complicações e com
satisfação elevada para os pacientes e os autores. Trinta e seis pacientes
foram submetidas à reconstrução mamária com a técnica descrita, com média de
idade de 59 anos. As complicações apresentadas foram necrose de complexo
areolopapilar, deiscência, seroma, hematoma, liponecrose, deslocamento do
implante e trombose venosa profunda. Nenhuma paciente teve necessidade de
resgate da reconstrução ou apresentou recidiva da neoplasia mamária durante
o período do estudo.
Conclusão: Trata-se de técnica que preserva a pele da mama em sua parte inferior, com
baixa possibilidade de deiscência ou extrusão do implante nesta região,
proporcionando uma dupla proteção deste implante com o músculo peitoral
maior nos dois terços superiores e o retalho cutâneo-dermogorduroso no terço
inferior, caracterizando um "dual-plane".
Palavras-chave: Mastectomia; Neoplasias da mama; Mamoplastia
INTRODUCTION
The National Cancer Institute1 shows that 57,960 new cases of breast cancer were expected in Brazil in 2016 (25% of all cancers), accounting for 14,388 deaths of women in 20132. Excluding non-melanoma skin tumors, this cancer type is the most frequent among women in Brazil and the second in the world. A surgical approach is the main step in treating this cancer.
There are several options for breast reconstruction, and the indications for each option have undergone some changes and evolutions. The use of flaps in association with implants has been gaining attention. The main limitation of using implants for breast reconstruction is inadequate soft tissue coverage. With the advent of skin-sparing mastectomy and subcutaneous mastectomy, immediate reconstruction, either with implants or autologous tissues, is now considered as the first option in selected patients3,4.
Despite the high efficiency of these techniques, some local complications persist with greater incidence in immediate reconstructions. Persisting complications include increased risk of seroma formation, skin necrosis, extrusion and exposure of the implants, and malfunctioning valve and leakage/deflation when expanders are used.
The advent of skin-sparing mastectomy and immediate reconstruction has allowed plastic surgeons and mastologists to achieve better results with all types of breast reconstruction. As skin flaps resulting from mastectomy have become more reliable, the rate of complications has decreased, and the satisfaction of the medical staff has increased.
Approximately 30 years after introducing the skin-sparing techniques, we are now able to face the challenge of achieving good aesthetic results in immediate reconstructions without significantly interfering with adjuvant therapy5-8.
The technique presented here consists of creation of an arcuate and versatile incision from the lateral end to the medial end of the breast, passing tangentially below, in the middle, or above the nipple-areola complex (NAC). This provides a wide exposure of the breast tissue to be accessed and reconstructed during mastectomy, allowing good coverage for the implant and the necessary skin adjustment for each case.
The technique is indicated for tumors of the upper or central quadrants, in which the skin in the lower quadrants can be partially preserved. The NAC could be preserved following the oncological treatment indicated by the mastologist. It could also be maintained, when possible, by maintaining the superior or inferior pedicle, depending on each case. An important aspect for the success of the technique described here is a good integration between the mastectomy team and the plastic surgery team.
OBJECTIVE
This article aims at presenting an approach for immediate breast reconstruction by creating a cavity in the retropectoral plane in the upper pole and in the mixed subcutaneous (subcutaneous and skin) plane in the lower pole, resulting in a less morbid dual-plane cover. This result is achieved by creating an arcuate incision in the breast, with the main advantage of protecting the prosthesis in cases of dehiscence of the wound, since the suture line of the deep plane (pectoralis muscle with subcutaneous flap) does not coincide with the skin suture.
METHODS
Our approach consists of creation of an arcuate and versatile incision from the lateral end of the breast sulcus to its medial end, passing tangentially below, in the middle, or above the NAC. This provides a wide exposure of the breast tissue to be accessed and reconstructed during mastectomy, providing an adequate coverage for the implant and the necessary skin adjustment for each case.
The technique is indicated for tumors of the upper or central quadrants, where the skin in the lower quadrants can be preserved. The NAC could be preserved following the oncological treatment indicated by the mastologist. It could also be maintained, when possible, by maintaining the superior or inferior pedicle, according to each case.
This retrospective study analyzed the medical records of patients operated for breast cancer from 2012 to 2016 at the Plastic Surgery Service of the senior author’s private clinic at Daher Hospital and the Armed Forces Hospital in Brasília, DF. All patients with tumors located in the upper quadrants of the breast and those with skin adequate to cover the lower third of the implant were selected.
Patients were selected after a joint analysis with the mastology team. All patients had a small amount of fat tissue in the lower quadrants; thus, an agreement on the maintenance of this tissue was necessary. Thus, tumor location, absence of microcalcifications or other suspicious changes, and blood viability of the flap from the perforators of the 6th and 7th intercostal spaces9 and the subdermal plexus of the upper abdomen were carefully analyzed.
All patients were operated on in a hospital setting, were discharged after surgery, and received a prescription for antibiotic prophylaxis with cefadroxil 500 mg every 12 hours, a vacuum suction drain, and analgesia; they were informed of the need for early ambulation. Enoxaparin sodium was only used for prophylaxis of deep venous thrombosis in selected cases; however, all mechanical preventive measures were routinely performed in all patients.
This research study followed the legal procedures determined by Resolution 196/96 of the National Health Council regarding research involving human beings. Data collection and analysis started soon after the examination and approval by the Research Ethics Committee of the Armed Forces Hospital (registration number 54273216.3.0000.0025 and opinion number 1,549,048).
Operative technique
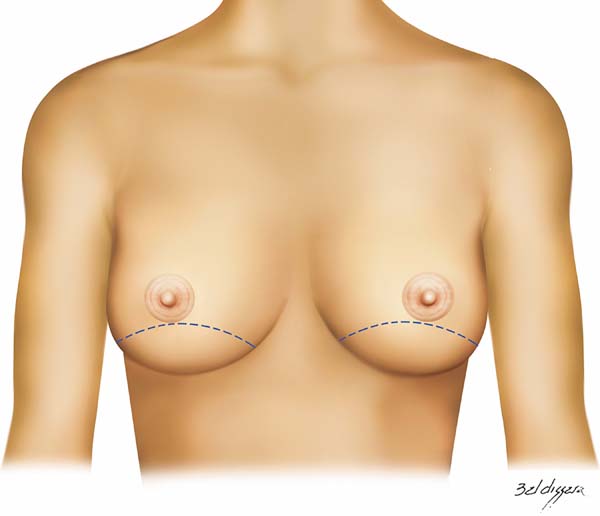
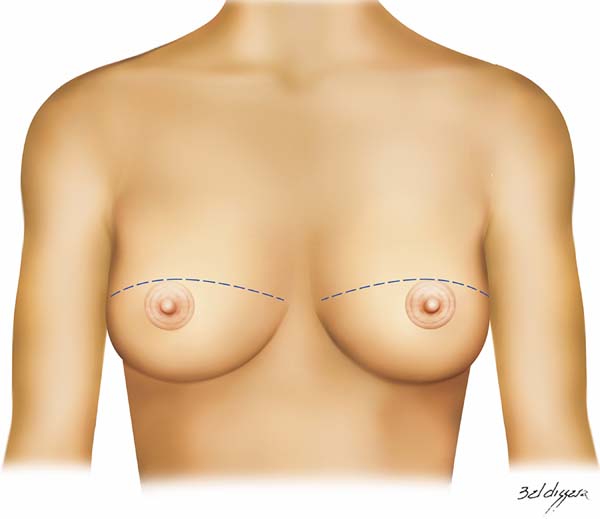
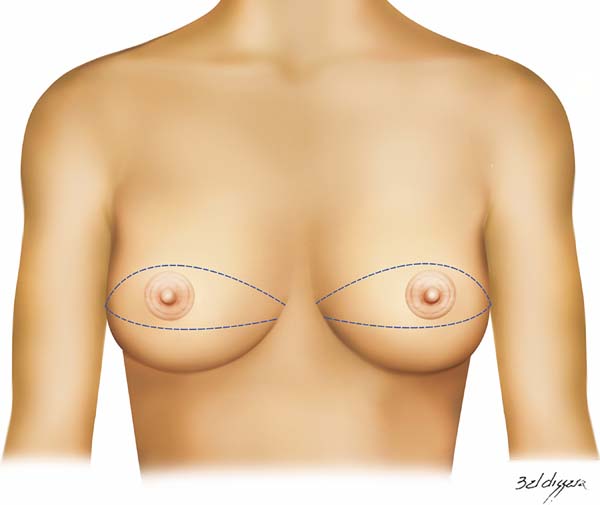
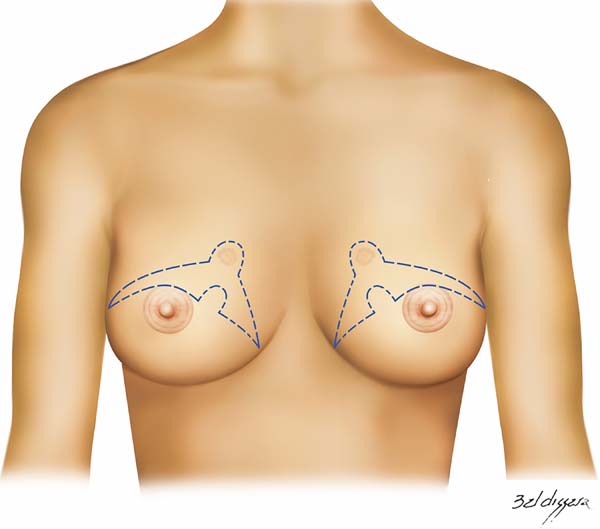
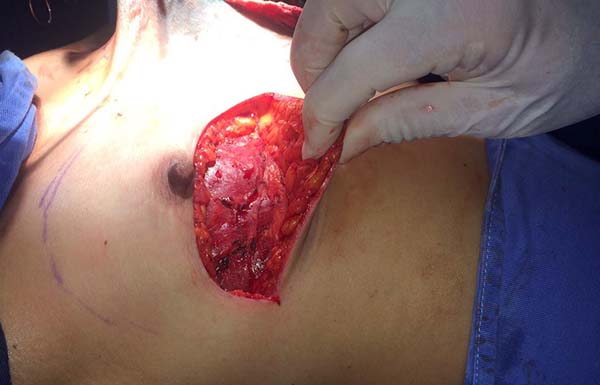
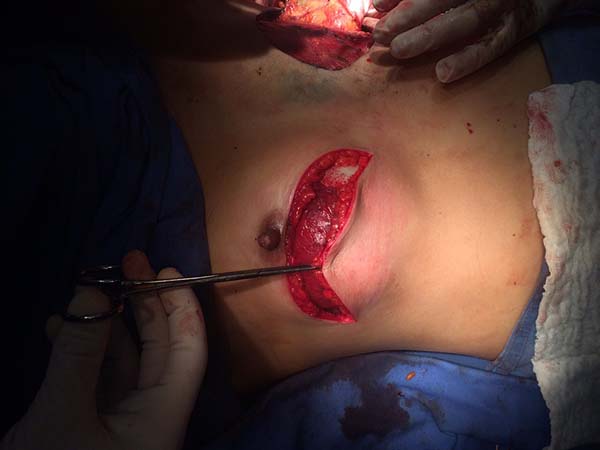
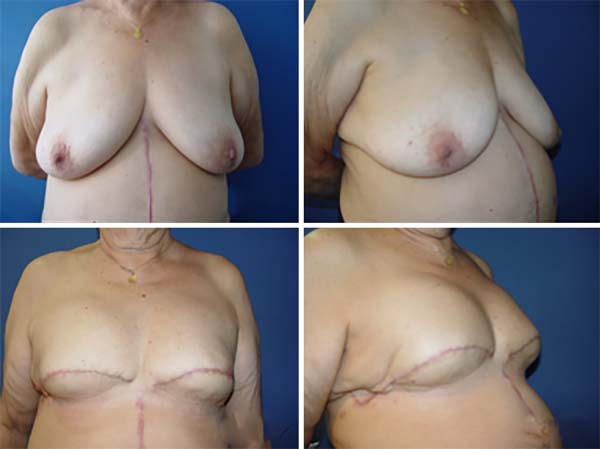
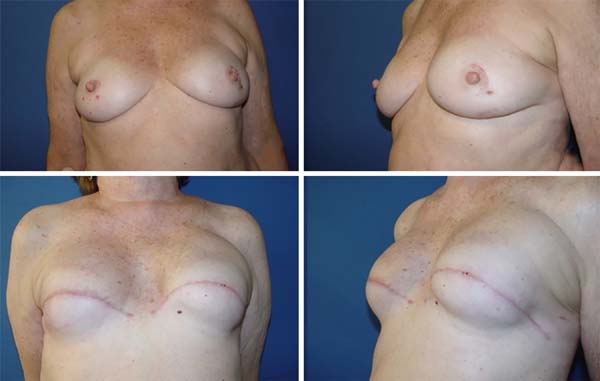
The technique used consists of creating an arcuate incision in the breast from the lateral end to the medial part of the sub-mammary sulcus, following the marks shown in figure 1. It can be performed bilaterally and simultaneously and for therapeutic or risk-reducing purposes. The incision may be created across the infra-areola or supra-areola region, outlining the upper or lower NAC or removing it completely. Further marking on the excess skin may be created if skin removal is required during mastectomy (Figures 2 to 4).
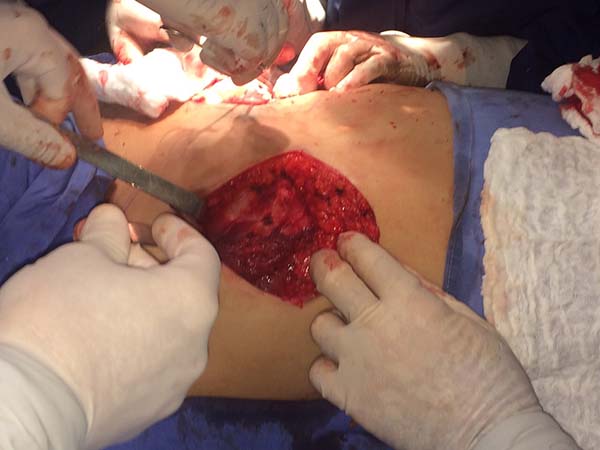
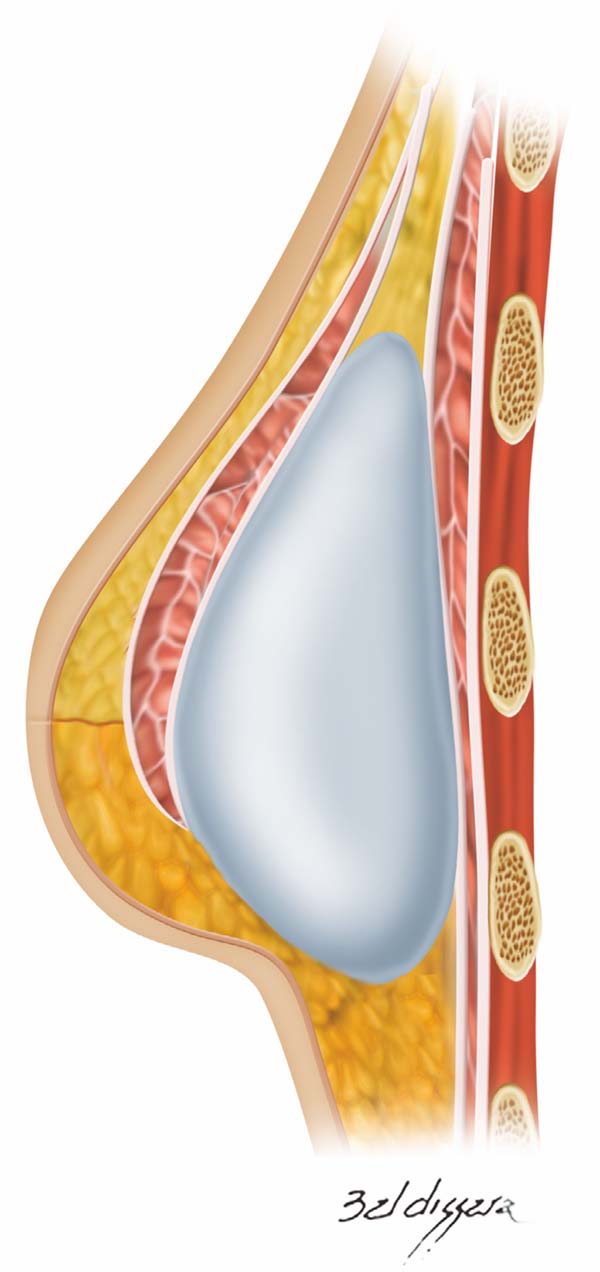
The entire lower base of the breast skin was maintained intact. The dermis-fat flap had a minimum thickness of 1.5 to 2 cm (Figure 5) and was placed by the plastic surgeon or at least under his/her supervision. Subsequently, mastectomy was performed by the mastology team through a wide incision that facilitates the procedure. After mastectomy, the plastic surgeon prepared the flap of the pectoralis major muscle by releasing it upon insertion of the costal insertion (Figure 6) near the rectus sheath aponeurosis and the costal insertion located at the junction of the 4th and 5th ribs.
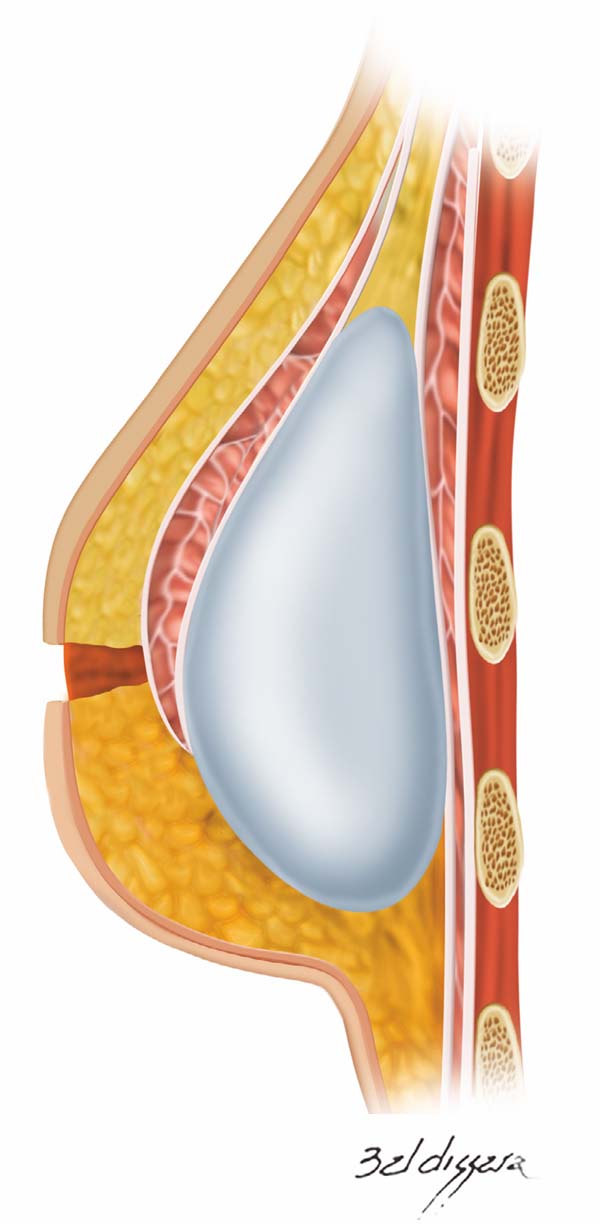
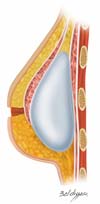
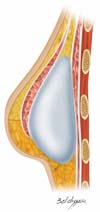
Once the retromuscular cavity was dissected, the silicone implant was inserted and covered by the pectoralis major muscle in the higher two-thirds and the dermis-fat flap in its lower third (Figure 7). The caudal border of the major pectoralis muscle was then sutured approximately 2 or 3 cm from the lower border of the dermis-fat flap (Figure 8), ensuring that this suture line does not coincide with the skin incision line (Figure 9) and thus providing greater protection for the implant in the case of dehiscence of the external suture.
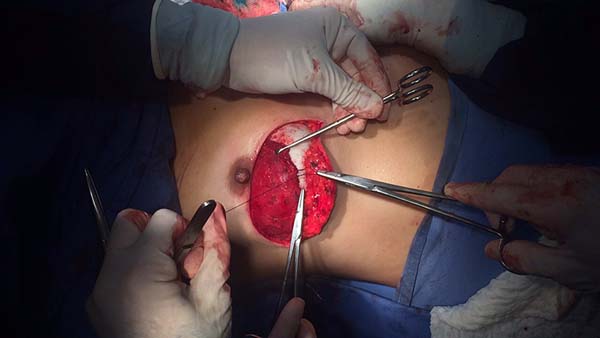
The skin of the upper portion of the breast covered the pectoral muscle, and its lower border was sutured at the upper edge of the lower flap, where the necessary adjustments and resections of skin excess were made (Figure 10). Thereafter, the implant remained well protected in a “dual plane” positioning (Figure 11). A vacuum suction drain was used routinely, and skin synthesis was conducted in two or three suture planes.
The implants used were round shaped for slim patients and regular shaped for patients with thicker adipose tissue; in other cases, they were selected according to the thickness left by the breast cancer specialist at the time of mastectomy. The volume selected was near 60% of the volume desired by the patient and/or surgeon. The NAC, when preserved, can be maintained with superior pedicle, inferior pedicle, or free graft, always according to mastology.
RESULTS
The mean patient age was 59.57 years. Ductal carcinoma in situ and infiltrating ductal carcinoma were the histological types found in the biopsy. High blood pressure, diabetes, and obesity were the most common comorbidities. Smoking was reported by five patients (15.78% of the total). Neoadjuvant therapy was required in two patients (5.5%) and adjuvant therapy in four (11.11%). Postoperative radiotherapy was indicated and performed in four patients (11.11%) without significant complications.
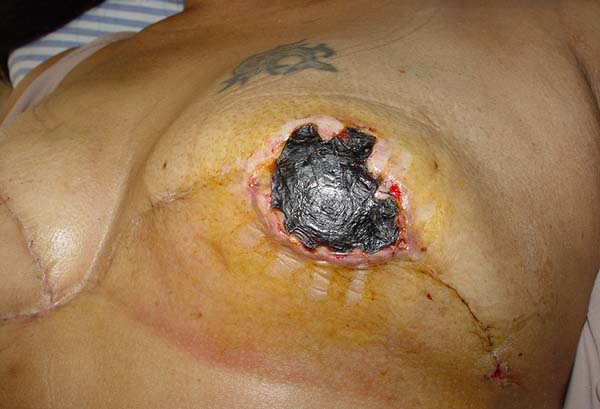
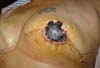
The complications in the 36 patients operated were NAC necrosis (one case; 2.7% - Figure 12), skin dehiscence without implant exposure (two cases; 5.5%), seroma (two cases; 5.5%), hematoma (one case; 2.7%), liponecrosis (one case; 2.7%), superior displacement of the implant (one case; 2.7%), and deep vein thrombosis (two cases; 5.5%). No case of implant extrusion or necrosis of the flap skin was observed. No patient showed recurrence of breast neoplasia in the breasts treated with the proposed technique during the study period.
Necrosis was treated by area delimitation followed by debridement and reconstruction of the NAC in a second procedure. The case that showed hematoma was correlated with the postoperative use of enoxaparin sodium, which is not part of the routine procedures performed by the senior author.
DISCUSSION
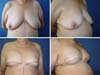
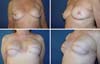
The results regarding oncological and esthetic safety were quite satisfactory. The approach was also safe regarding possible complications of adjuvant treatments (Figures 13 and 14). The rate of complications obtained by the author while using the technique described here was lower than that reported for most reconstruction techniques using implants10,11.
As breast reconstruction techniques have evolved and became more sophisticated, the expectation for better aesthetic results by the patients followed. They want plastic surgeons to provide a reconstructed breast with a more natural shape and texture and with minimal scarring.
With the increasing use of immediate reconstruction techniques, the search for adequate coverage of the implants has increased. This may result in larger scars distant from the primary site in the case of reconstructions using a large dorsal or rectus abdominis flap. The technique presented in this paper results in a single arcuate scar in the lower quadrants of the breast, leading to a less stigmatizing aspect.
NAC necrosis was observed in 1.9% of the cases analyzed in the study by Eskenazi12, in which several breast reconstruction techniques were compared; it was found in 2.7% in our study. Seroma was observed in 4% of the cases in the same study against 5.5% observed in our study. In Eskenazi’s study, 7.1% of the cases had skin flap necrosis against none of our cases.
The recovery of the patients subjected to this technique is quick because the implant is not fully covered with the pectoralis major muscle, which contributes to a lower perception of postoperative pain.
The dual plane is not an unprecedented technique. However, in the technique presented, the pectoral muscle covers less than two thirds of the implant, leaving it freer. This facilitates natural ptosis, in addition to reducing the risk for superior implant migration, a common complication in reconstructions and aesthetic surgeries with placement of the implant in the retropectoral plane. Furthermore, there is a lower risk of implant extrusion owing to the interposition of the viable tissue between the implant and the skin13, and no incision is created in the lower portion of the breast14.
This technique provides adequate coverage for the implant in patients with recommendations for adjuvant therapy, providing adequate implant protection and reducing the risk of extrusion and capsular contracture or need for implant removal, complications found in some patients undergoing breast reconstruction using implants15. If the patient develops capsular contracture, reoperation is simple and with satisfactory results in most cases.
The pectoralis muscle flap and lower cutaneous pedicle technique has more advantages than techniques using acellular dermal matrix16,17, since it does not require the use of a matrix, which, in addition to having a high cost18,19, has a greater risk of infection20,21.
The technique presented here, compared with the pectoralis muscle with lower dermis-fat pedicle technique14,22, has the advantage of leaving a thinner flap without glandular tissue, which favors greater oncological safety. Furthermore, it frees the patient from extensive scars and risk of dehiscence with implant exposure and necrosis in the lower portion of the breast.
Consideration should also be given to maintaining the integrity of the upper abdomen, thoracolateral, large dorsal, and TRAM flaps for the possible need for salvage surgery.
CONCLUSION
The technique proposed by the authors is a viable alternative for selected cases and may be part of the portfolio of techniques available for breast reconstruction, since it is easy and quick to perform, has low morbidity and complication rates, is similar to other techniques, and provides satisfactory esthetic results and oncological safety.
Moreover, it provides a broad field for mastectomy, allowing easy access to sentinel lymph node or axillary dissection, without the need for further incisions.
COLLABORATIONS
|
OMC |
Final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents. |
|
IRJ |
Analysis and/or interpretation of data; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents. |
|
LGM |
Writing the manuscript or critical review of its contents. |
|
DASS |
Writing the manuscript or critical review of its contents. |
|
LMCD |
Writing the manuscript or critical review of its contents. |
|
MCAG |
Writing the manuscript or critical review of its contents. |
|
GCS |
Writing the manuscript or critical review of its contents. |
|
LDPB |
Writing the manuscript or critical review of its contents. |
|
PJC |
Writing the manuscript or critical review of its contents. |
|
MVSO |
Analysis and/or interpretation of data; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents. |
|
LCE |
Conception and design of the study; writing the manuscript or critical review of its contents. |
REFERENCES
1. Brasil. Ministério da Saúde. Instituto Nacional do Câncer José Alencar Gomes da Silva (INCA). Mama: Estimativa de novos casos. Rio de Janeiro: INCA; 2018. [acesso 2018 Maio 22]. Disponível em: http://www2.inca.gov.br/wps/wcm/connect/tiposdecancer/site/home/mama
2. Brasil. Ministério da Saúde. Portal da Saúde. SIM-Sistema de Informações de Mortalidade. DATASUS. [acesso 2018 Maio 22]. Disponível em: http://www2.datasus.gov.br/DATASUS/index.php?area=060701
3. Roostaeian J, Pavone L, Da Lio A, Lipa J, Festekjian J, Crisera C. Immediate placement of implants in breast reconstruction: patient selection and outcomes. Plast Reconstr Surg. 2011;127(4):1407-16. PMID: 21460648 DOI: http://dx.doi.org/10.1097/PRS.0b013e318208d0ea
4. Cammarota MC, Ribeiro Junior I, Lima RQ, Almeida CM, Moura LG, Daher LMC, et al. Estudo do uso de pontos de adesão para minimizar a formação de seroma após mastectomia com reconstrução imediata. Rev Bras Cir Plást. 2016;31(2):158-65.
5. Spear SL, Spittler CJ. Breast reconstruction with implants and expanders. Plast Reconstr Surg. 2001;107(1):177-87. DOI: http://dx.doi.org/10.1097/00006534-200101000-00029
6. Slavin SA, Schnitt SJ, Duda RB, Houlihan MJ, Koufman CN, Morris DJ, et al. Skin-sparing mastectomy and immediate reconstruction: oncologic risks and aesthetic results in patients with early-stage breast cancer. Plast Reconstr Surg. 1998;102(1):49-62. PMID: 9655407 DOI: http://dx.doi.org/10.1097/00006534-199807000-00008
7. Singletary SE. Skin-sparing mastectomy with immediate breast reconstruction: the M. D. Anderson Cancer Center experience. Ann Surg Oncol. 1996;3(4):411-6. DOI: http://dx.doi.org/10.1007/BF02305673
8. Kroll SS, Ames F, Singletary SE, Schusterman MA. The oncologic risks of skin preservation at mastectomy when combined with immediate reconstruction of the breast. Surg Gynecol Obstet. 1991;172(1):17-20. PMID: 1985335
9. van Deventer PV, Graewe FR. The Blood Supply of the Breast Revisited. Plast Reconstr Surg. 2016;137(5):1388-97. PMID: 27119914 DOI: http://dx.doi.org/10.1097/PRS.0000000000002048
10. Camirand A, Doucet J, Harris J. Breast augmentation: compression--a very important factor in preventing capsular contracture. Plast Reconstr Surg. 1999;104(2):529-38. DOI: http://dx.doi.org/10.1097/00006534-199908000-00038
11. Salgarello M, Farallo E. Immediate breast reconstruction with definitive anatomical implants after skin-sparing mastectomy. Br J Plast Surg. 2005;58(2):216-22. DOI: http://dx.doi.org/10.1016/j.bjps.2004.06.033
12. Eskenazi LB. New options for immediate reconstruction: achieving optimal results with adjustable implants in a single stage. Plast Reconstr Surg. 2007;119(1):28-37. DOI: http://dx.doi.org/10.1097/01.prs.0000244744.27540.cc
13. Hartrampf CR, Bennett GK, Casas LA. Clinical analysis of results: Complications, prevention, management and salvage procedures. In: Hartrampf CR. Hartrampf's Breast Reconstruction with Living Tissue. New York. Raven Press; 1991.
14. Cosac OM, Curado DMdC, Cammarota MC, Daher JC, Esteves BP, Camara Filho JP, et al. Reconstrução mamária com retalho dermogorduroso de pedículo inferior associado ao músculo peitoral. Rev Bras Cir Plást. 2014;29(1):75-8.
15. Ching AW, Costa MP, Brasolin AG, Ferreira LM. Influência das complicações pós-operatórias no insucesso da reconstrução de mama imediata com implante de silicone. Rev Bras Cir Plást. 2015;30(2):182-9.
16. Weichman KE, Wilson SC, Weinstein AL, Hazen A, Levine JP, Choi M, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129(5):1049-58. PMID: 22544088 DOI: http://dx.doi.org/10.1097/PRS.0b013e31824a2acb
17. Weichman KE, Wilson SC, Saadeh PB, Hazen A, Levine JP, Choi M, et al. Sterile "ready-to-use" AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;132(4):725-36. PMID: 23783060 DOI: http://dx.doi.org/10.1097/PRS.0b013e31829fe35b
18. Jordan SW, Khavanin N, Fine NA, Kim JY. An algorithmic approach for selective acellular dermal matrix use in immediate two-stage breast reconstruction: indications and outcomes. Plast Reconstr Surg. 2014;134(2):178-88. DOI: http://dx.doi.org/10.1097/PRS.0000000000000366
19. Krishnan NM, Chatterjee A, Van Vliet MM, Powell SG, Rosen JM, Nigriny JF. A comparison of acellular dermal matrix to autologous dermal flaps in single-stage, implant-based immediate breast reconstruction: a cost-effectiveness analysis. Plast Reconstr Surg. 2013;131(5):953-61. PMID: 23629077 DOI: http://dx.doi.org/10.1097/PRS.0b013e3182865a24
20. Spear SL, Sher SR, Al-Attar A, Pittman T. Applications of acellular dermal matrix in revision breast reconstruction surgery. Plast Reconstr Surg. 2014;133(1):1-10. PMID: 24105085 DOI: http://dx.doi.org/10.1097/01.prs.0000436810.88659.36
21. Liu AS, Kao HK, Reish RG, Hergrueter CA, May JW Jr, Guo L. Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg. 2011;127(5):1755-62. PMID: 21228744 DOI: http://dx.doi.org/10.1097/PRS.0b013e31820cf233
22. Cosac OM, Costa LAC, Barros APGSH. Reconstrução mamaria bilateral com retalhos pediculados. In: Melega JM, Viterbo F, Mendes FH, eds. Cirurgia Plástica: Os princípios e a atualidade. Rio de Janeiro: Guanabara Koogan; 2011. p. 732-42.
1. Sociedade Brasileira de Cirurgia Plástica, São
Paulo, SP, Brazil.
2. Hospital das Forças Armadas, Brasília, DF,
Brazil.
3. Hospital Daher Lago Sul, Brasília, DF,
Brazil.
Corresponding author: Ismar Ribeiro
Junior
Av. Pau Brasil, lote 20 - Ed. Via Naturale, apt 2402-2
Brasília, DF, Brazil Zip Code 71926-000
E-mail: ismarjr@gmail.com
Article received: June 22, 2016.
Article accepted: May 17, 2018.
Conflicts of interest: none.





 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter