

Original Article - Year 2017 - Volume 32 - Issue 4
Reconstruction of the chest wall in extensive oncological resections
Reconstrução de parede torácica em extensas ressecções oncológicas
ABSTRACT
INTRODUCTION: In spite of advances in the diagnosis and treatment of patients with breast cancer, there are still cases that present with locally advanced disease. In this context, cancer control requires extensive resections and complex repair procedures. The participation of the plastic surgeon in the multidisciplinary cancer treatment teams has been increasingly important in both the planning and execution stages. The aim of this study is to prospectively analyze patients who underwent extensive chest resections and thoracic wall reconstruction with regard to their results and complications in the period 2014-2016.
METHODS: Prospective 6-month follow-up of 15 patients who underwent extensive oncological resections in the chest and reconstruction by the same plastic surgeon in public hospitals of São Paulo was done. The following data were collected: age, diagnosis, preoperative complaint, extent of resection area, and incidence of local complications.
RESULTS: Patients had a mean age of 49.2 years, the most common complaint was a papillary mass, the most common diagnosis was breast cancer (80%), the most resection area preserved was the muscular plane, and the mean resection area was 259.2 cm2. The V-Y latissimus dorsi myocutaneous flap was the most used, and the thoracoabdominal flap was the second. Epitheliolysis was detected in 13.3% of the patients, and partial necrosis less than 5% of the flap in 13.3%.
CONCLUSION: In the present study, patients with extensive cancers in the thorax presented a challenge for local reconstruction, requiring detailed preoperative planning and multiple options. The participation of the plastic surgeon in the treatment of these patients contributed to the reduction of morbidity rate and low rate of complications.
Keywords: Reconstructive surgical procedures; Breast cancers; Mammoplasty; Thoracic wall; Soft tissue cancer.
RESUMO
INTRODUÇÃO: Apesar do avanço no diagnóstico e no tratamento das pacientes com neoplasia mamária, ainda há casos que se apresentam como doença localmente avançada. Nesse contexto, o controle oncológico requer ressecções extensas e complexos procedimentos reparadores. A participação do cirurgião plástico nas equipes multidisciplinares de tratamento oncológico tem apresentado importância crescente tanto nas etapas de planejamento como de execução. O objetivo é analisar prospectivamente casos de pacientes com extensas ressecções no tórax e reconstrução de parede torácica quanto aos seus resultados e complicações no período de 2014-2016.
MÉTODOS: Seguimento prospectivo por 6 meses de 15 casos de pacientes submetidas a extensas ressecções oncológicas no tórax e reconstrução por um mesmo cirurgião plástico em hospitais da rede pública de São Paulo-SP. Foram coletados os dados: idade, diagnóstico, queixa pré-operatória, extensão da área de ressecção e incidência de complicações locais.
RESULTADOS: As pacientes tinham idade média de 49,2 anos, a queixa mais comum era massa papável, o diagnóstico mais comum era neoplasia da mama (80%), a maioria das ressecções preservou o plano muscular e a área média de ressecção foi de 259,2 cm2. O retalho miocutâneo do grande dorsal em V-Y foi o mais utilizado, e o toracoabdominal foi o segundo. Epiteliólise foi detectada em 13,3% das pacientes e necrose parcial menor que 5% do retalho em 13,3%.
CONCLUSÃO: Na presente casuística, as pacientes portadoras de neoplasias extensas no tórax representaram um desafio para a reconstrução local, necessitando de planejamento pré-operatório minucioso e com múltiplas opções. A participação do cirurgião plástico no tratamento desses casos contribuiu para redução de morbidade e demonstrou baixo índice de complicações.
Palavras-chave: Procedimentos cirúrgicos reconstrutivos; Neoplasias da mama; Mamoplastia; Parede torácica; Neoplasias de tecidos moles.
Breast cancer is the most common cancer in women in Brazil and worldwide, excluding non-melanoma skin cancer1,2. With early diagnosis and the advances in treatment, the survival of these patients is growing every year, even in more advanced stages; thus, functional and social rehabilitation through the reconstruction of the breast and chest wall are important aspects of the treatment of these patients1.
Despite population-based screening campaigns and educational efforts, locally advanced cancer, although not representing the majority of breast cancer cases, can comprise up to 20-25%3. In populations assisted by the public health service, these cases are still frequent due to the difficulty in accessing referral centers and early diagnosis. They are classified as stage III, in which patients have a large (2-5 cm or more) compromised breast, with invasion of the skin or chest wall and/or regional lymph nodes, without distant metastasis4.
Naturally, these situations present the greatest challenges to the plastic surgeon in the planning and execution of the local reconstruction. Even when disease-free survival may not be the main goal of treatment, as in very advanced tumors, reconstruction is essential to confer local adjuvant treatment conditions (closing bloody areas for radiotherapy) and increase quality of life in the survival period5,6.
The advancement of chest wall reconstruction techniques has a strong and intimate relationship with the treatment of breast cancer by mastectomy, from the time of Halsted, in 1882, in which wounds were left to heal by second intention, until the appearance of muscle flaps in 1896 with Tansini and, more recently, the use of microsurgery7.
The technical difficulty of thoracic wall reconstruction is dictated for the most part by the variability in the extent of resections and, consequently, the unpredictable nature of the extension of the resulting defects. The main point is the definition of the need for reconstruction of the costal bone framework, indicated when four or more ribs are resected or there is a lateral defect of more than 5 cm, situations in which the stability of the thoracic scaffold is compromised8.
The need for coverage of important structures and extensive areas makes muscle or myocutaneous flaps the most common options for the reconstruction of extensive oncological defects in the thorax7,9. The most commonly used flaps are the latissimus dorsi myocutaneous flap, transverse rectus abdominis myocutaneous (TRAM) flap, and pectoralis major myocutaneous flap associated with skin grafting10. In addition, as long as there are no synthetic materials or bone exposure, local fasciocutaneous flaps, such as thoracoepigastric and thoracoabdominal flaps, are also valuable options. Random cutaneous flaps, such as the rhomboid and advancement flaps, and even skin grafts are also used in particular cases in which the simplicity of the restorative strategy is essential.
The participation of the plastic surgeon in the treatment of these patients has been of increasing importance within multidisciplinary teams, involving mastologists, thoracic surgeons, and oncologists. The idea that the presence of the restorative professional in the preoperative therapeutic planning could compromise the oncological rigor has been abandoned. In fact, it can be recognized that the plastic surgeon, with the ability to perform large restorative interventions with versatility, allowed the treatment of lesions previously considered unresectable and contributed to reduce the recovery time of these patients, besides providing them greater comfort and quality of life7,11.
OBJECTIVE
The aims of this study are to prospectively analyze cases of patients subjected to thoracic wall reconstruction after extensive oncological resections regarding the surgical strategies adopted and their results and complications and to define the role of the plastic surgeon in the therapeutic planning of patients with locally advanced breast cancer.
METHODS
Fifteen patients submitted to extensive resections of thoracic lesions and reconstruction of the chest wall, treated by surgery, and followed up prospectively in the postoperative period in two reference services of the public health network in São Paulo, Brazil, were evaluated in the preoperative period.
The procedures of this study followed the principles of Helsinki, and the patients signed an informed consent form for inclusion in the study group.
All patients were operated by the same plastic surgeon (MMAS) in conjunction with the Thoracic Surgery and/or Mastology teams. They had postoperative follow-up of 3-6 months.
Standardized photographic documentation was performed pre-, intra-, and postoperatively, according to the clinical conditions of the patients.
During follow-up, the following data were collected: age, initial symptom, diagnosis, extent of resection area of cancer lesion, depth of resection (remaining plane), technique used for reconstruction, incidence of complications associated with surgical intervention, and death in 6 months.
The data were analyzed through descriptive statistics and summary measures, with the numerical variables being expressed as means and categorical variables as percentages.
Surgical technique
The surgical techniques of the main flaps used in this study are described below:
V-Y latissimus dorsi myocutaneous flap
Marking was performed with the patient in orthostatic position, making a triangle whose base is the lateral margin of the anterior thoracic defect. The sides of the triangle are formed by lines that start from the upper and lower margins of the defect in the anterolateral thoracic wall and converge to the posterior midline of the thorax. Convergence can occur before the midline (in case of minor defects to rebuild) or beyond (extensive defects or need for "ear" compensation).
The surgical technique consisted of positioning the patient in lateral decubitus opposite the donor area and with an abducted upper limb. The incisions were up to the muscular plane where the entire extension of the latissimus dorsi is dissected from the iliac crest to its insertion in the humerus, which can be released in order to add amplitude in its rotation arc. Skeletonization of the thoracodorsal pedicle is undesirable but may also be required to increase the arc of rotation. The donor area was closed primarily with suture by planes and adhesion sutures and submitted to vacuum drainage. After transposition, the flap was attached to the pectoral muscles. The planes were closed.
Thoracoabdominal flap
Marking was performed with the patient in orthostatic position. The incision should be marked from the lower lateral angle of the thoracic defect and follow the lower curve in the axillary midline. The patient was placed in the horizontal dorsal decubitus position on the surgical table, and the dissection proceeded from the incision in the medial and inferior direction in a subfascial plane, preserving the superior epigastric perforators. The flap was then advanced superiorly toward the defect. Vacuum drainage was performed in all patients, and the flap was sutured by planes.
RESULTS
The study sample included 15 female patients aged 32 to 66 years (mean age 49.2 years).
Preoperative complaints were palpable masses in 46.7% of cases, palpable and ulcerated masses in 46.7%, and skin retraction in 6.6%. All tumors were unilateral.
The patients underwent surgery for resection of malignant cancers of the thorax, two of them by thoracic surgery because they were cases in which the invasion of the chest wall in its bony components was evident in the preoperative clinical evaluation. The others were submitted to oncological resection by the Mastology team.
All patients were submitted to preoperative evaluation by the multidisciplinary team, and surgical planning was carried out, including primary and secondary reconstruction options, maintaining the principle of performing the simplest reconstruction possible and sparing more elaborate options for possible situations of relapse.
Most patients (80%) had invasive ductal carcinoma of the breast at stage III, indicating a locally advanced tumor and/or lymph node involvement. Two of these patients had relapsed lesions. The remaining patients (20%) presented soft tissue sarcomas of the thorax, all of them being high-grade sarcomas in the histological analysis.
Surgeries were done under general anesthesia, and there were no intraoperative complications. Most of the resections (86.7%) provided intact or partially preserved muscular plane beds. Only two patients (13.3%) had resection of the thoracic wall musculature throughout their thickness and bony elements of the costal grid. The latter had reconstruction in conjunction with the team of thoracic surgery using polypropylene mesh and one of them needed metal bars.
The extent of the bloody areas left by the resections had a mean area of 259.2 cm2. The length ranged from 3-30 cm (mean 16.2 cm), and the width ranged from 8-25 cm (mean 16 cm).
Among the reconstruction options, local flaps (thoracoabdominal fasciocutaneous, rhomboid, and skin advancement) were used in 6 patients (40%). Among the muscle flaps, 60% of the flaps used were the V-Y latissimus dorsi skin island (46.7%), TRAM (6.7%), and vertical myocutaneous flaps of the rectus abdominis with bilobed skin island (6.7%).
Patients received intravenous antibiotic therapy intraoperatively and for seven days postoperatively. They were submitted to suction drainage in the donor and recipient areas in all the flaps, except rhomboid and local advancement flaps.
During the postoperative follow-up, two patients (13.3%) presented epitheliolysis in the early postoperative period, with healing by second intention and excellent late outcome. Two patients (13.3%) presented with partial necrosis (less than 5%) and underwent chemical debridement with ointment and follow-up for second intention healing, with excellent results after 2 months of follow-up.
All patients followed up in this study received adjuvant chemotherapy and/or radiotherapy, and one patient (6.7%) died before the end of the 6-month follow-up. All patients who underwent thoracic reconstruction completed the successful follow-up.
Table 1 shows the data presented above, which characterize the sample and the reconstructive strategies. Table 2 presents a summary of means and standard deviations of the numerical variables.
Figures 1, 2, 3, 4, and 5 show the percentage distributions of symptoms, diagnosis, deep defect plan, chosen flaps, and complications.
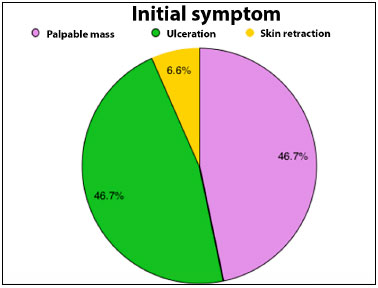
Figure 1. Pie graph showing percentage distribution of the main initial symptoms presented by the patients in the multidisciplinary evaluation for the treatment of cancer.
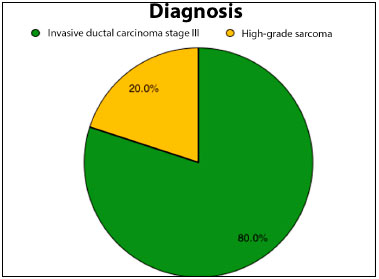
Figure 2. Pie graph showing percentage distribution of anatomopathological diagnosis of cancer lesions.
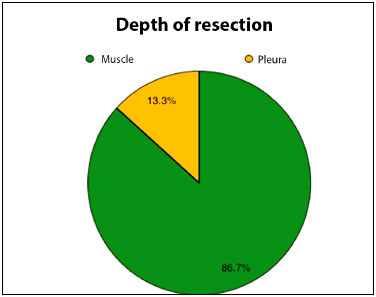
Figure 3. Pie graph demonstrating percentage distribution of depth of resection of cancer lesions.
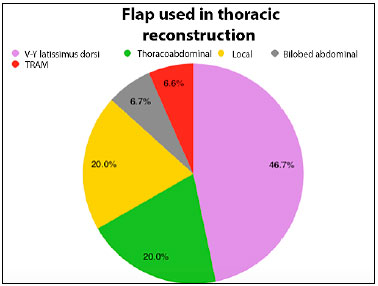
Figure 4. Pie graph showing percentage distribution of the flaps performed for thoracic wall reconstruction in the patients studied. TRAM, transverse rectus abdominis myocutaneous flap.
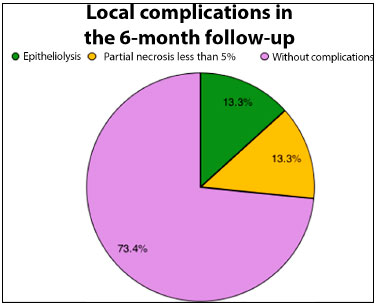
Figure 5. Pie graph showing percentage distribution of local complications detected in the 6-month follow-up of patients submitted to extensive oncological resections and chest wall reconstructions.
Figures 6 to 14 show photographs of patients in the study.
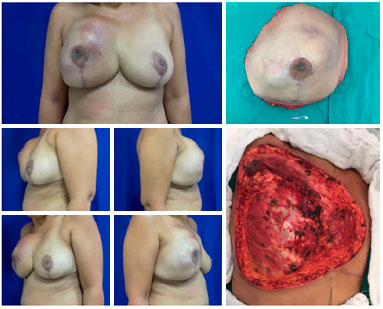
Figure 6. Preoperative view of patient with recurrent soft tissue sarcoma (left). Resected part intraoperatively weighing 2.3 kg (upper right). Tumor resection bed measuring 25 × 20 cm (lower right).
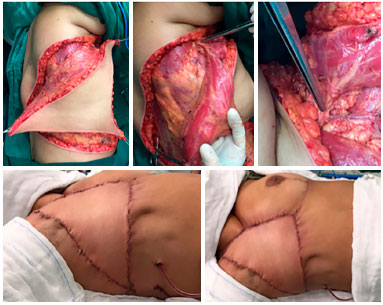
Figure 7. Intraoperative view of patient with recurrent soft tissue sarcoma. Myocutaneous flap preparation of the V-Y latissimus dorsi skin island and highlight of the dissected pedicle (upper line). Final aspect of the flap already sutured in the recipient area (bottom right line).
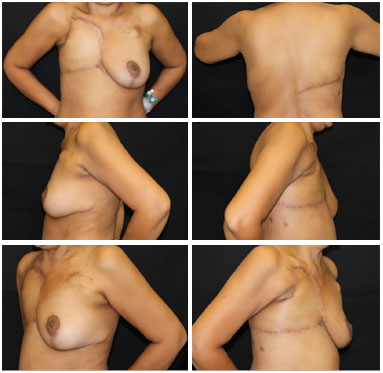
Figure 8. Postoperative 6 months view, patient with recurrent soft tissue sarcoma with excellent outcome of the V-Y latissimus dorsi flap, with no complications or signs of relapse.
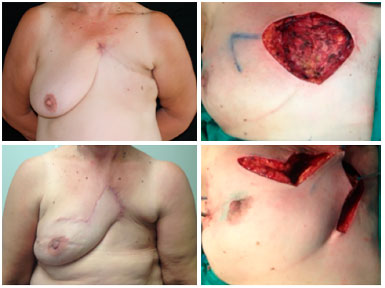
Figure 9. Preoperative view of a patient with tumor recurrence of breast cancer on the chest skin (upper left). Intraoperative view with local reconstruction through rhomboid flap - marking (upper right) and transposition (lower right). Postoperative 6 months view of rhomboid flap with optimal result (lower left), without complications or signs of relapse.
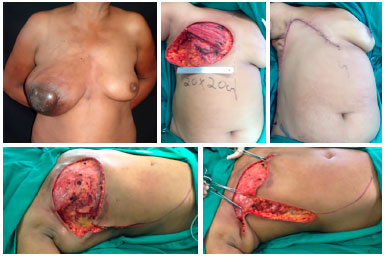
Figure 10. Preoperative view of a patient with locally advanced breast cancer (upper left). Intraoperative view with dimensions of the resection bed (upper central). Final aspect of the reconstruction with the thoracoabdominal flap (upper right). Intraoperative view with demarcation of incisions (lower left) and demonstration of the advancement of the flap toward the receptor area (lower right), determining factor for the correct extension of the dissection.
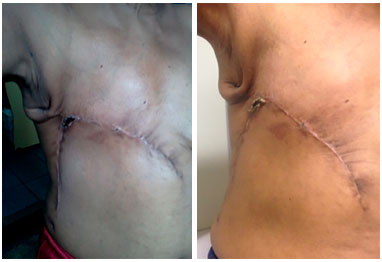
Figure 11. Postoperative view of 1 month (left) and 45 days (right) of a patient with locally advanced breast cancer submitted to reconstruction with a thoracoabdominal flap, which evolved with partial necrosis of less than 5% of the area. Good evolution of the area of necrosis with delimitation, chemical debridement, and healing by second intention.
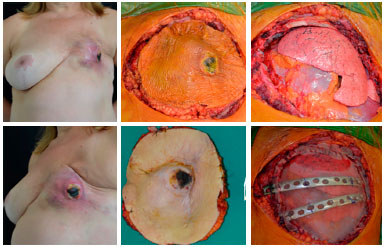
Figure 12. Preoperative view of a patient with ulcerated soft tissue sarcoma (left). Intraoperative view with delimitation of the resection (upper central), resection bed (upper right), and aspect of the resected part (lower central), and reconstruction of thoracic scaffold by the Thoracic Surgery team.
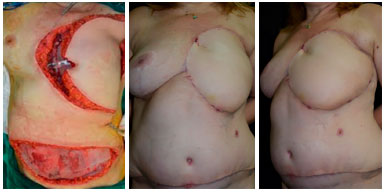
Figure 13. Intraoperative view of a patient with ulcerative soft tissue thoracic sarcoma demonstrating the position of the transverse rectus abdominis myocutaneous (TRAM) flap in the receptor area. Postoperative view of 3 months with good aspect of the flap, without signs of relapse or complications.
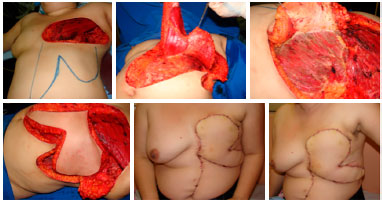
Figure 14. Intraoperative view of a patient with locally advanced breast cancer showing abdominal bilobed flap (upper left) with muscular component in its vertical lobe (upper central) and fasciocutaneous in its horizontal lobe (upper right). Intraoperative view illustrating the end of the preparation of the bilobed flap (lower left). Postoperative of 3 months in frontal view (lower central) and left oblique (lower right) with good appearance, without signs of recurrence or complications.
DISCUSSION
Until recently, a large proportion of extensive tumors were considered non-resectable, and advanced breast tumors were considered contraindications for the immediate reconstruction of the breast. The main reasons for these were the concern with the control of margins and the increase in the wound area with the possibility of complications5. The delay in wound healing can have negative repercussions at the time of initiation of adjuvant therapy.
In case of sarcomas, the second most frequent tumor in this sample, surgical treatment consisting of resection with wide margins is the factor most associated with a good prognosis of long-term survival. The reconstruction of the chest wall with its bone and soft tissue components, in turn, is a factor of great importance for the overall success of the treatment12.
The planning of cancer treatment and repair of the thoracic wall together should involve a careful analysis of the quantity of resected soft tissue, bone, and cartilaginous elements, type of tumor, autologous and synthetic materials available, neighborhood flaps and distance in good vascularization conditions, maintenance of functional aspects (such as the pulmonary expansion) and aesthetics, when possible11. The plastic surgeon must be a professional qualified to perform multiple complex repairs (from smallest to largest) and must be ready to tailor therapeutic proposals to intraoperative conditions, which often impose the need for adaptation or change of plans.
The importance of preoperative multidisciplinary assessment including that by the plastic surgeon is highlighted by several authors as essential to the success of complex treatments involving large resections and reconstructions3,7,11,13. For Franco et al.,11 the presence of the plastic surgeon, from the planning stage to the implementation of the treatment, contributes to the preservation of important structures for rehabilitation without damage to oncological principles.
Chen et al.7, from the University of Wisconsin, USA, compiled 91 cases of chest reconstruction, and malignant tumors were the main cause of extensive resections of the extrapleural thoracic wall. They considered the cautious but liberal use of muscular flaps to be of utmost importance for the success of such reconstructions. As in the present study, the latissimus dorsi myocutaneous flap was the most frequent flap used, but two flaps were needed for reconstructing the majority of their cases.
Marcondes et al.14, as in this study, described a series of cases with closure of the chest wall by multiple strategies. The sample was composed of slightly younger patients, with an average age of 44.9 years, and all had locally advanced breast cancer. Three fasciocutaneous flaps, 2 skin grafts, and 6 myocutaneous flaps were used, the most widely used being the rectus abdominis flap. Although reconstruction strategies have a different distribution compared to the present study, the results were also described as satisfactory. The choice of reconstruction method is influenced by many intraoperative factors, by the patients' own conditions and surgeon's preference and experience.
The latissimus dorsi myocutaneous flap was the most common flap used in this study, being the option chosen for 7 (46.7%) of the patients. In spite of its description dating back to the nineteenth century by Tansini, the latissimus dorsi myocutaneous flap remains the main option for reconstruction in many cases for several reasons, including the ease of dissection, constancy and safety of its vascular pedicle, and a large arc of rotation, being able to reach cervical, anterior, and dorsal thoracic regions and can be enlarged by humeral disinsertion up to 5-10 cm.
The disadvantages pointed out for this flap are the need for intraoperative lateral decubitus for dissection, high seroma index in the donor area, operative wound dehiscence, and apparent/hypertrophic scarring in the donor area10. The general and specific functional capacity of the ipsilateral upper limb to the large dorsal flap does not change after surgery, and the quality of life improves after the procedure in populations with non-advanced tumors15,16.
Maia et al.17, in 2011, proposed a modification of the location of the skin island in the latissimus dorsi myocutaneous flap. With an elegant anatomical study associated with a case report, they showed that there is reliable vascularization for the preparation of the skin island on the lower third of the muscle, so the arc of rotation reaches central areas of the anterior chest with ease. In 2006, Woo et al.18 proposed a modification in the flap design with a curvilinear design that recruited more skin for closure.
However, the modification responsible for the higher impact in the indication of the latissimus dorsi myocutaneous flap was performed by Micali and Carramaschi19, in 2001. The authors proposed a modification of the skin island flap to a triangular shape with the apex in the posterior midline and base facing the defect with closure in V-Y. This modification enabled the recruitment of a much higher skin extension of the latissimus dorsi myocutaneous flap and broaden its scope of coverage for upper areas of the chest wall, where the TRAM and the thoracoabdominal flap, other important options, do not reach. The V-Y skin island flap allowed the primary closure of the donor area and the appreciable increase in the size of defects that the flap could rebuild. After its revelation, other authors published their positive experience with the flap13,20.
Munhoz et al.13 reported a series of 25 patients treated with radical mastectomy and reconstruction with the V-Y latissimus dorsi myocutaneous flap with a mean age of 54.8 years. As in the present study, all patients had a successful reconstruction and were not delayed at the start of adjuvant therapy. Minor complications developed in two patients, but none required increased hospitalization time or readmissions.
Fasciocutaneous flaps, such as the thoracoabdominal flap, the second most common flap in this study, are based on the superficial vascularization of perforating arteries. There is a richly vascularized area in this region, allowing the production of long, thick, resistant, and safe flaps. They can be used with medial or lateral pedicles depending on the need to cover the defect. The thoracoabdominal flap has a medial base and uses superior epigastric artery perforators. It has a good indication when the need for medial advancement is lower than the lateral21.
Park et al.21 described a series of 24 cases in which the closure of extensive areas of mastectomy was done exclusively with thoracoabdominal, thoracoepigastric, or bilateral advancement fasciocutaneous flaps. After a follow-up of 14 months, 36% reported complications, the majority small and with resolution upon conservative treatment. A shorter surgical time, less blood loss, and a shorter postoperative hospital stay are known advantages of fasciocutaneous flaps22.
Particularly in cases of extensive resections, such as those described in this study, manipulation of the thoracic and axillary regions is frequent. Thus, verifying the integrity of the vascular pedicles is fundamental for the success of the flaps8,13. In the present study, all latissimus dorsi myocutaneous and TRAM flaps had their main pedicle intact. The other flaps had no pedicles dissected.
As with the integration of the principles of plastic surgery in the planning of tumor resections of breast cancer, making the programming of breast excision and reconstruction parts of an integrated process, large centers have begun a thoracoplastic approach for tumors with extension to the thoracic wall. Thus, the team's plastic surgeon performs the incision markings and the initial dissection of muscular planes in order to preserve important structures for the restorative surgery without compromising the oncological result.
In a series of cases at the University of Philadelphia, with 45 patients, 14 of whom were treated with a thoracoplastic approach; no differences were observed regarding complications, tumor recurrence, or reconstruction success compared to those who had a usual approach by the thoracic surgery team. As in the present study, this series of cases was composed of patients with primary tumors of the chest wall or breast, the latissimus dorsi myocutaneous flap was the most used, and the rate of complications was low. Only patients with loss of bone structure had systemic complications. The participation of the plastic surgeon was considered an important factor for the success of the treatment8.
CONCLUSION
In the present sample, patients with extensive neoplastic disease of the thorax represent a challenge for reconstruction after being submitted to extensive resections.
The participation of a team of plastic surgery was beneficial for the success of such procedures and low rate of complications in the described sample.
Preoperative planning with multiple options stratified in complexity helps the intraoperative decisions and contributes to lower morbidity rate in the reported cases.
COLLABORATIONS
MMAS Compilation of photographic documentation; literature review; final approval of the manuscript; study conception and design; execution of operations and/or experiments;
drafting of manuscript or critical review of its content.
MSN Final approval of the manuscript; study conception and design; execution of operations and/or experiments.
ATL Final approval of the manuscript; compilation of photographic documentation; execution of operations and/or experiments.
PAPG Final approval of the manuscript; execution of operations and/or experiments.
LMF Final approval of the manuscript; study conception and design.
REFERENCES
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. DOI: http://dx.doi.org/10.3322/caac.21332
2. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva - INCA. Estimativa 2016: Síntese de resultados e comentários. [acesso 2016 Out 11]. Disponível em: http://www.inca.gov.br/estimativa/2016/sintese-de-resultados-comentarios.asp
3. D'Aiuto M, Cicalese M, D'Aiuto G, Rocco G. Surgery of the chest wall for involvement by breast cancer. Thorac Surg Clin. 2010;20(4):509-17. DOI: http://dx.doi.org/10.1016/j.thorsurg.2010.09.001
4. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva - INCA. Condutas do INCA/MS 2001. Câncer de Mama. [acesso 2016 Out 11]. Disponível em: http://www.inca.gov.br/rbc/n_47/v01/pdf/normas.pdf
5. Lee MC, Newman LA. Management of patients with locally advanced breast cancer. Surg Clin North Am. 2007;87(2):379-98. DOI: http://dx.doi.org/10.1016/j.suc.2007.01.012
6. Drake DB, Oishi SN. Wound healing considerations in chemotherapy and radiation therapy. Clin Plast Surg. 1995;22(1):31-7.
7. Chen JT, Bonneau LA, Weigel TL, Maloney JD, Castro F, Shulzhenko N, et al. A Twelve-Year Consecutive Case Experience in Thoracic Reconstruction. Plast Reconstr Surg Glob Open. 2016;4(3):e638. DOI: http://dx.doi.org/10.1097/GOX.0000000000000603
8. Basta MN, Fischer JP, Lotano VE, Kovach SJ. The thoracoplastic approach to chest wall reconstruction: preliminary results of a multidisciplinary approach to minimize morbidity. Plast Reconstr Surg. 2014;134(6):959e-67e. PMID: 25415119 DOI: http://dx.doi.org/10.1097/pRS.0000000000000734
9. Larson DL, McMurtrey MJ. Musculocutaneous flap reconstruction of chest-wall defects: an experience with 50 patients. Plast Reconstr Surg. 1984;73(5):734-40. PMID: 6718572 DOI: http://dx.doi.org/10.1097/00006534-198405000-00003
10. Skoracki RJ, Chang DW. Reconstruction of the chestwall and thorax. J Surg Oncol. 2006;94(6):455-65. PMID: 17061266 DOI: http://dx.doi.org/10.1002/jso.20482
11. Franco D, Tavares Filho JM, Cardoso P, Moreto Filho L, Reis MC, Boasquevisque CH, et al. Plastic surgery in chest wall reconstruction: relevant aspects - case series. Rev Col Bras Cir. 2015;42(6):366-70. DOI: http://dx.doi.org/10.1590/0100-69912015006003
12. Foroulis CN, Kleontas AD, Tagarakis G, Nana C, Alexiou I, Grosomanidis V, et al. Massive chest wall resection and reconstruction for malignant disease. Onco Targets Ther. 2016;9:2349-58.
13. Munhoz AM, Montag E, Arruda E, Okada A, Brasil JA, Gemperli R, et al. Immediate locally advanced breast cancer and chest wall reconstruction: surgical planning and reconstruction strategies with extended V-Y latissimus dorsi myocutaneous flap. Plast Reconstr Surg. 2011;127(6):2186-97. PMID: 21617452 DOI: http://dx.doi.org/10.1097/pRS.0b013e318213a038
14. Marcondes CA, Pessoa SGP, Pessoa BBGP, Dias IS, Ribeiro NP. Estratégias em reconstruções de tórax pós-ressecções extensas de tumores de mama localmente avançados: uma série de 11 casos. Rev Bras Cir Plást. 2015;30(3):339-44.
15. Guerreiro V, Sabino Neto M, Dutra AK, Ferreira LM. Capacidade funcional de pacientes submetidas a reconstrução mamária com retalho musculocutâneo de latíssimo do dorso. Rev Bras Cir Plást. 2013;28(3):367-74.
16. Dutra AK, Neto MS, Garcia EB, Veiga DF, Netto MM, Curado JH, et al. Patients' satisfaction with immediate breast reconstruction with a latissimus dorsi musculocutaneous flap. J Plast Surg Hand Surg. 2012;46(5):349-53. DOI: http://dx.doi.org/10.3109/2000656X.2012.704726
17. Maia M, Oni G, Wong C, Saint-Cyr M. Anterior chest wall reconstruction with a low skin paddle pedicled latissimus dorsi flap: a novel flap design. Plast Reconstr Surg. 2011;127(3):1206-11. PMID: 21088645 DOI: http://dx.doi.org/10.1097/pRS.0b013e318205f2f7
18. Woo E, Tan BK, Koong HN, Yeo A, Chan MY, Song C. Use of the extended V-Y latissimus dorsi myocutaneous flap for chest wall reconstruction in locally advanced breast cancer. Ann Thorac Surg. 2006;82(2):752-5. PMID: 16863813 DOI: http://dx.doi.org/10.1016/j.athoracsur.2005.07.030
19. Micali E, Carramaschi FR. Extended V-Y latissimus dorsi musculocutaneous flap for anterior chest wall reconstruction. Plast Reconstr Surg. 2001;107(6):1382-90. DOI: http://dx.doi.org/10.1097/00006534-200105000-00010
20. Luz DP, Lobo CAH, Hiraki P, Okada A, Montag E, Ferreira MC. Retalho miocutâneo de latíssimo do dorso em V-Y para reconstrução de grandes defeitos torácicos extensos. Rev Bras Cir Plast. 2010;25(3 Suppl.1):64.
21. Park JS, Ahn SH, Son BH, Kim EK. Using local flaps in a chest wall reconstruction after mastectomy for locally advanced breast cancer. Arch Plast Surg. 2015;42(3):288-94. DOI: http://dx.doi.org/10.5999/aps.2015.42.3.288
22. Persichetti P, Tenna S, Cagli B, Scuderi N. Extended cutaneous 'thoracoabdominal' flap for large chest wall reconstruction. Ann Plast Surg. 2006;57(2):177-83. DOI: http://dx.doi.org/10.1097/01.sap.0000215253.54577.28
1. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
2. Universidade Federal de São Paulo, São Paulo, SP, Brazil
Institution: Universidade Federal de São Paulo, São Paulo, SP, Brazil.
Corresponding author:
Mayara Mytzi de Aquino Silva
Rua Napoleão de Barros, 740 - 2º andar - Vila Clementino
São Paulo, SP, Brazil - Zip Code 04023-062
E-mail: mmytzi@yahoo.com.br
Article received: October 12, 2016.
Article accepted: July 10, 2017.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter