

Special Article - Year 2017 - Volume 32 -
Practical criteria for a safer liposuction: a multidisciplinary approach
Critérios práticos para uma lipoaspiração mais segura: uma visão multidisciplinar
ABSTRACT
In 2012, the Research Group on Body Contouring Surgery of the Brazilian Society of Plastic Surgery conducted an extensive study on the safety of liposuction before the initiation of the course of the Group, held in 2013 in Rio de Janeiro during the 50th Brazilian Congress of Plastic Surgery, with the presence of the illustrious Dr. Yves Gérard Illouz and a large number of participants. The authors performed a review and update of the safety parameters, incorporating recent advances and systematizing relevant information for the execution of a safer liposuction. With the collaboration of anesthesiologist, intensive care physician, and vascular surgeon, all with extensive experience in supporting the procedure, new practical guidelines were presented for a safer liposuction on the preoperative, transoperative, and postoperative periods.
Keywords: Lipectomy; Patient safety; Intraoperative complications; Postoperative complications
RESUMO
O Capítulo de Cirurgia do Contorno Corporal da Sociedade Brasileira de Cirurgia Plástica realizou em 2012 um amplo estudo dos parâmetros de segurança para a realização de uma lipoaspiração, antecedendo ao curso do Capítulo, realizado em 2013, no Rio de Janeiro, durante o 50º Congresso Brasileiro de Cirurgia Plástica, com a presença do ilustre Dr. Yves Gérard Illouz e grande número de participantes. Os autores realizaram uma revisão e atualização destes parâmetros, incorporando recentes avanços e sistematizando de forma prática as informações relevantes para a realização de uma lipoaspiração mais segura. Com a colaboração de anestesiologista, intensivista e cirurgião vascular, experientes no suporte ao procedimento, são apresentadas novas orientações práticas para o pré-operatório, trans e pós-operatório de uma lipoaspiração mais segura.
Palavras-chave: Lipectomia; Segurança do paciente; Complicações intraoperatórias; Complicações pós-operatórias.
Liposuction is the second most common surgical procedure in Brazil, according to a survey conducted by Datafolha in 20091. Previous studies reported that in 2011, liposuction was the most performed plastic surgery in Brazil, with a total of 211,108 procedures2. In a worldwide survey promoted by the International Society for Aesthetic Plastic Surgery, 1,614,031 among 11,599,336 esthetic surgical procedures performed across the globe in 2013 were liposuction, and Brazil ranked first in performing these procedures3.
Mortality due to liposuction ranges from 1 for every 5,000 patients (American Society of Plastic Surgeons, 1998) to as low as 1 for every 47,425 in cases in which liposuctions are performed by plastic surgeons with a recognized background in esthetic procedures (American Society for Aesthetic Plastic Surgery [ASAPS], 2001)4. In the year 2000, an ASAPS survey5 reported that the mortality from liposuction was 19.1 for every 100,000 procedures and that pulmonary thromboembolism (PTE) was the most common cause of death.
The significant contribution of liposuction to plastic surgery has been quickly assimilated by many plastic surgeons worldwide. Despite many innovations, from the abandoned dry technique to laser liposuction, the principle of skin retraction over a bed formed from hollow tunnels has been maintained over the years. However, indications, safety standards, and technical refinements have undergone several changes because of the creativity, innovation, and experience of renowned surgeons and particularly because of legal and normative considerations, which vary considerably in different countries.
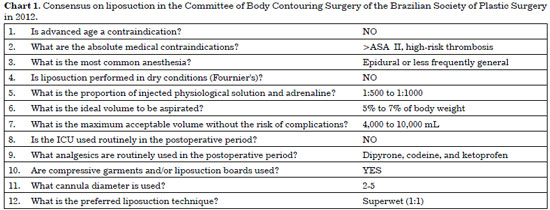
In 2012, the Research Committee on Body Contouring Surgery of the Brazilian Society of Plastic Surgery (Sociedade Brasileira de Cirurgia Plástica [SBCP]) sent a questionnaire with 34 questions to the Scientific Council of the Committee. The responses obtained in consensus are summarized in Chart 1.
The problems of overweight and obesity have reached epidemic proportions worldwide. The World Health Organization estimates that in 2025, approximately 2.3 billion individuals across the globe will be overweight and more than 700 million will be obese. A survey conducted by the Ministry of Health of Brazil in 2014 indicated that 52.5% of the population in Brazil is above the ideal weight (43% in 2006), and 17.9% is obese (body mass index [BMI] greater than or equal to 30 kg/m2)7.
The increase in the number of individuals with obesity in Brazil and overseas, limited efficacy of dietary treatments, and morbidity inherent to bariatric surgery have contributed to the increased number of requests for liposuction as a treatment option. Most of these patients are rejected by most plastic surgeons for such a procedure under the premise that liposuction should only be performed for the removal of localized fats. However, the leading author reassessed these indications considering cases of patients with obesity, for whom liposuction was used as a coadjuvant to nutritional treatments and physical exercise programs.
The use of the superwet technique (a ratio of the infiltrate to the total aspirate of 1:1) has been recommended by most authors as an adequate technique for safe liposuctions8. However, plastic surgeons do not limit the amount of fat to be aspirated by the amount of tissue infiltration throughout the procedure in daily practice; this indicates that surgeons have unequivocal certainty regarding the execution of superwet liposuction only at the end of the procedure when the content of the liposuction flask is weighed and when compared with the total amount of infiltrate placed in the subcutaneous tissues during surgery. This paradox requires particular attention to the postoperative period so that excessive fluid replacement does not lead to acute pulmonary edema or insufficient fluid replacement to hypovolemic shock.
The maximum volume to be aspirated safely during liposuction has been a matter of debate9. According to the pioneer of liposuction, Yves Gerard Illouz, the aspirated volume should not exceed 6% to 8% of body weight or no more than 30% of the patient's body surface10.
The resolution of the Federal Medical Council (Conselho Federal de Medicina [CFM]) of Brazil determined that the aspirated volume should not exceed 7% of body weight when using the infiltrative technique or 5% when using the non-infiltrative technique. The same resolution also determined that the total liposuction area should not exceed 40% of the body surface area regardless of the technique used11.
The results of a survey conducted by the SBCP branch in São Paulo in 2014 on the total aspirated volume during liposuction indicated that 47.65% of surgeons use the limit of 7% of body weight; 39.60% use the limit of 5%; and 8.05% use the limit of 3%12. The United States does not have a well-established rule: Some States (i.e., California, Florida, Kentucky, New York, Ohio, and Tennessee) have determined a maximum limit of 1,000-5,000 cc for liposuction. When performed together with other surgeries, the state of Florida restricts the aspirated volume to 1,000 cc and Tennessee to 2,000 cc8. The BMI has been correlated with the maximum volume of fat tissues that can be safely aspirated and corresponds to 100 cc for each BMI unit9.
The use of blood and blood derivatives, even patients' own blood, always implies potential risks to the patient. These risks and the convenience of indicating other elective surgeries together with liposuction in esthetic surgeries need to be reviewed.
The prevention of thrombosis and fat embolism requires efficient, normative, and if possible, specific protocols for liposuction considering the severity of these conditions.
The authors compiled updated norms for the indication and execution of a safer liposuction in a practical and objective manner based on a review of the recommendations of the 2012 Research Committee of Body Contouring Surgery of the SBCP; the current bibliographic review is based on the known pathophysiology and professional experience of plastic surgeons, anesthesiologists, vascular surgeons, and intensive care physicians, all with extensive experience in supporting the procedure and its complications.
DISCUSSION
An extensive and comprehensive review of the surgical safety of liposuction, morbidity, and mortality was conducted by Gomes4 in 2003 in Brazil. In 2009, the Evidence-Based Patient Safety Advisory for Ambulatory Surgery by Haeck et al.8 in the United States devoted 17 pages to aspects relevant to patient safety during and after liposuction considering evidence-based criteria.
Indications of liposuction
Plastic surgeons may contraindicate liposuction to healthy patients in cases of obesity and flaccidity, which may occur simultaneously.
Although preliminary studies indicate reduction of cardiovascular risk and decrease in blood pressure and insulin level after liposuction, liposuction has not been considered a standard procedure for the treatment of obesity13. Some authors consider that obesity is responsible for the maintenance of a subclinical inflammatory state associated with leukocytosis.
However, there is no evidence to date that liposuction improves the lipid profile or the levels of leptin, adiponectin, resistin, interleukin, and C-reactive protein or decreases the levels of tumor necrosis factor alpha in individuals with obesity14. The eligibility for liposuction should be carefully evaluated in patients with a BMI above 30 kg/m2 because these patients have a lower rate of scarring and increased rate of infection, deep vein thrombosis (DVT), and sleep apnea15.
Patients with obesity present psychological benefits from liposuction. In addition, these patients often seek plastic surgeons after repeated failure of diets, physical exercise programs, and use of anorectic drugs. The first author, following the classic dictates recommended by Illouz, contraindicated liposuction in these cases but questioned this contraindication after treating a young patient with obesity weighing 118 kg (Figures 1A-1D) with a liposuction aspirate of 4,500 mL (Figures 1E-1G). This patient maintained the achieved outcome for more than 3 years and presented a dramatic change in lifestyle, including the practice of sports, and a remarkable improvement in his psychosocial profile.

Figure 1. A 118-kg patient subjected to adjuvant liposuction as a palliative treatment for obesity. A, B, C, and D: Preoperative appearance; E, F, and G: Postoperative appearance after adjuvant liposuction of 4,500 mL.
Other obesity cases treated by the same surgeon, with aspirate volumes of 5,000 to 6,000 mL, have shown partial relapses in some cases and success in others. Although liposuction is not a usual treatment for obesity8, this experience, although subjective, suggests that in patients who fully understand the adjuvant nature of the treatment and the esthetic limitation of the result (the outcome in these patients is not as good as in lean patients with localized fat), liposuction can and should be indicated in cases of obesity. However, the surgeon should inform the relatives regarding these issues and establish legal protection by a specific informed consent, explaining the coadjuvant nature of the esthetic procedure.
Another situation involves patients with pronounced flaccidity in the abdominal region, when they do not accept abdominal dermolipectomy/lipoabdominoplasty after considering the resulting scar, possible future pregnancy, or lack of time for an adequate postoperative healing period (Figures 2A-2C).
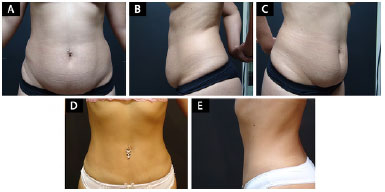
Figure 2.
A 27-year-old patient with abdominal lipodystrophy associated with flaccidity subjected to adjuvant liposuction for not accepting the scars of a dermolipectomy. A, B, and C: Preoperative appearance; D and E: Postoperative appearance.
The same situations described for patients with obesity are valid for these patients: We cannot predict the degree of cutaneous retraction and the suitability of the procedure against the esthetic expectations of the patient. However, we can indicate liposuction in some cases considering the level of understanding of the patient and the acceptance of the limitations, with gratifying results for the patient and the surgeon (Figures 2D-2E).
Preoperative period: evaluation, warnings, fasting, and use of chronic drugs
The preanesthetic evaluation should be performed by an anesthesiologist according to Resolution 1363/93 of the CFM. In fact, this evaluation is the beginning of the anesthetic procedure because it reduces perioperative morbidity and mortality, allows the anesthesiologist to plan for the most appropriate anesthesia for the case, and establishes a personal relationship with the patient, contributing to patient reassurance regarding the scheduled anesthesia induction and surgery.
In preanesthetic anamnesis, the patient should be questioned regarding allergies (e.g., latex and iodine) and abuse of licit drugs (e.g., benzodiazepines, cigarettes, and alcohol) and illicit drugs, without moralism. The patients who smoke should stop smoking 6 to 8 weeks before surgery; if this is not possible, discontinuation is recommended at least 24 hours before surgery to reduce carboxyhemoglobin levels and increase the amount of oxygen released into the tissues. The surgeon should inquire about the date of last menstruation considering that several patients who undergo this procedure are of a childbearing age.
Caution should be taken with frequent users of appetite suppressants among the patients who are eligible for liposuction because these drugs act on the central nervous system, releasing noradrenaline into the synaptic cleft or inhibiting the reuptake of noradrenaline at the presynaptic terminal. The increase in the levels of sympathetic neurotransmitters in the synaptic cleft causes sympathetic hyperstimulation, which leads to hypertension, tachycardia, arrhythmias, and severe complications.
The use of medications, such as amfepramone, fenproporex, and sibutramine, should be suspended at least 14 days before the scheduled surgery. We also recommend interruption of the use of monoamine oxidase (MAO) inhibitor antidepressants 15 days before surgery.
Although antiplatelet agents are usually discontinued 7 to 10 days before surgeries that present a high risk of bleeding, there is a controversy as to the suitability of acetylsalicylic acid (ASA) interruption in patients who use it prophylactically because its abrupt discontinuation may contribute to the prothrombotic environment common in the postoperative period. However, there is no evidence of the benefit of suspending ASA therapy in patients who use it prophylactically in surgeries with a low risk of bleeding16, such as liposuction.
The use of herbal medicines, such as ginseng, gingko biloba, and ginger, should be suspended 7 days before surgery because of the potentially higher risk of bleeding during surgery and anesthetic procedure. Patients with diabetes who use oral hypoglycemic agents should discontinue this treatment to avoid hypoglycemia during the fasting period. Regular insulin can be used if necessary. Patients with insulin-dependent diabetes should adjust their dose before surgery considering the number of meals that will not be ingested; however, this treatment should not be interrupted.
The use of chronic medications, such as antihypertensive drugs, lipid-lowering drugs, bronchodilators (including inhaled beta agonists and leukotriene inhibitors), non-MAO inhibitor antidepressants, anticonvulsants, and anti-thyroid drugs should be continued even on the day of surgery. Chronic corticosteroid users should replace their drugs with hydrocortisone in the intraoperative period to avoid adrenal insufficiency. Chart 2 shows the use of some drugs and potential interactions between drugs administered in the perioperative period17-21.
We should assess the presence of pre-existing diseases and history of anesthetic complications, including nausea, vomiting, post-spinal anesthesia headache, postoperative pain, and other complications. The possibility of anesthesia-induced malignant hyperthermia (incidence of 1 per 50,000 adult patients) should also be assessed during anamnesis, and if suspected, the standard diagnosis is performed via muscle biopsy using the caffeine halothane muscle contracture test because the creatine phosphokinase dosage is elevated in only 50% of the relatives of the affected individuals and is not unique to malignant hyperthermia22.
After thorough physical examination and definition of surgical risk (established by the American Society of Anesthesiologists), the anesthesiologist can prescribe preanesthetic medications and recommendations on fasting: After several changes in the recommendations, the current fasting recommendation is of at least 8 hours before surgery23-26. The generalization of fasting is more prudent, safe, and practical than the individualization of the recommended food products to be ingested.
Transoperative period: techniques, infiltrations (lidocaine and adrenaline), maximum volume, and change in decubitus
Dry liposuction, which was recommended by Pierre Fournier and later abandoned in favor of the tumescent technique, presented a blood loss of 20% to 45% of the aspirate. The superwet technique8 (1980), in which 1 to 2 mL of physiological solution with adrenaline is infiltrated for every 1 mL of aspirate, yields a blood loss of 1% to 2% of the aspirate and is the technique most commonly used in the clinical setting.
The tumescent technique, proposed by Klein in 1985, infiltrates 3 to 4 mL of a solution containing 0.025-0.1% lidocaine and adrenaline (1:1,000,000) per mL of aspirate. The main criticism of this technique is that the firm consistency of the skin prevents the surgeon from "embracing the cannula" with the non-dominant hand. Failure to comply with this rule may lead to an inadvertent perforation of the abdominal wall, pleura, or even the inferior lumbar triangle and severe damage to the internal organs. Another important aspect is that 70% of the infiltrate is transferred to the intravascular space in 2 to 10 hours, and an excessive infiltrate leads to acute pulmonary edema8.
Lidocaine is relatively lipid soluble and metabolized by the hepatic microsomal system. The binding of this drug to subcutaneous fat and the relatively poor vascularization under the skin delay its systemic absorption. However, there is a risk of toxicity 6 to 14 hours (or even 20 hours) after drug administration if the maximum recommended doses are not respected.
However, even in adequate doses, it is important to remember that the excretion of lidocaine is decreased in liver diseases, during reduced hepatic flow caused by the associated adrenaline, in cases of hypoproteinemia and during the use of medications, such as propranolol and cimetidine. When combined with adrenaline, the maximum safe dose of lidocaine for skin anesthesia is 7 mg/kg. Considering the effect of adrenaline, low absorption of this lidocaine in the fat tissues, and the simultaneous aspiration, the dose of lidocaine was polemically increased to 35 mg/kg by some authors8.
Chart 3 shows the effects of the plasma peaks of lidocaine. Llanos et al.27 observed that lidocaine combined with tumescent infiltration significantly increased the occurrence of anemia in the postoperative period. Considering all these aspects and the existence of many effective analgesics that can be used postoperatively, we do not see any justification for the use of lidocaine in liposuction under epidural or general anesthesia.
Although up to 10 mg of adrenaline can be administered safely8, it is prudent not to exceed 0.07 mg/kg and always consider the following contraindications: in cases in which halogenated anesthetics are used, severe arterial hypertension, pheochromocytoma, hyperthyroidism, cardiopathies, and peripheral vascular diseases8. In the case of the use of halogenated anesthetics, it is recommended to reduce the adrenaline dose from 0.07 mg/kg to 0.05 mg/kg because this drug combination increases the risk of arrhythmias.
A vital aspect of liposuction is the full understanding of the physiological consequences and potentially severe risks related to patient positioning, particularly during a spinal block. In the horizontal supine position, the influence of gravity on the vascular system is minimal. The intravascular pressure from the head to the feet minimally varies compared with the mean pressures close to the heart.
In the prone position, the pressure gradients in the blood vessels are minimal because of the peripheral vasodilatation promoted by the spinal block if the legs remain essentially horizontal. For this reason, the possibility of severe hypotension or even cardiac arrest during changes in decubitus should always be considered.
The anesthesiologist recommends handling the patient with firm, slow, and stepwise movements: First, the professional should move the patient laterally on the surgical table and should then make the careful rotation of the patient to ventral decubitus. Despite the scarce literature on the subject, we recommend the administration of a small dose of ephedrine (5 mg IV) or etilefrine (1 mg IV) immediately before the change in decubitus to help avoid blood pressure reduction.
The CFM and SBCP, through Resolution 1711 of December 10, 200311, limited the aspirated volume to a maximum of 7% of the weight or 40% of the body surface area with the infiltrative technique and without the coincidence of these parameters. However, in this criterion, the said resolution considered only the fatty fraction (supernatant) in the flask.
Although these criteria are normative, the authors usually consider the total aspirated volume present in the bottle because of the lack of studies that unequivocally demonstrate the convenience of considering only the aspirated fatty fraction relative to the electrolyte loss, despite the performed infiltration. The 7% body weight index, although consensual, is empirical and therefore was not obtained using evidence-based criteria.
Postoperative period: same-day discharge associated with the aspirated volume, electrolyte replacement, use of blood and blood products, thrombosis, fat embolism, and combinations of surgical procedures
The generic criteria for hospital discharge listed by CFM Resolution No. 1886 of 200828 included an adequate level of consciousness, stability of vital signs (blood pressure, pulse rate, and temperature) for at least 1 hour (in addition to presenting a blood pressure compatible with preoperative levels and patient age), absence of nausea and vomiting, absence of dyspnea, ability to swallow and cough, ability to ambulate, minimal bleeding, and absence of severe pain or urinary retention.
In addition to these criteria, each case should be individualized in liposuction29, remembering to avoid the use of lidocaine in the infiltration and observe the delayed peaks of this drug when it is used (Chart 3) and other specific conditions of the procedure. The leading author has eventually performed liposuctions of up to 5,000 mL with hospital discharges on the same day. These patients underwent liposuction under the following conditions: maximum aspiration of 5% of body weight, under the superwet technique in the early morning, without lidocaine, and under epidural anesthesia. These patients were discharged from the clinic 8 hours after the procedure, with all mentioned discharge criteria achieved and without any complications.
Electrolyte replacement
At present, we use oral replacement with isotonic solutions together with intravenous hydration when indicated, which is similar to fluid replacement therapy in burned patients. It has been a consensus to maintain a urinary output of 1 mL/kg/hour30 or a residual volume of 90-140 mL/kg8,30 (the residual volume is calculated by subtracting from the total volume of infiltration plus physiological solution received IV during the procedure, the sum of 30% of the aspirate plus the intraoperative diuresis).
For patients with an infiltration to aspirate ratio of 1:1, only 1 mL/kg/hour is sufficient in the fasting period (usually 3 hours). For patients with an aspirate higher than the infiltrate, this surplus volume is provided by the intravenous replacement of saline or Ringer's solution postoperatively (in fact, only 70% of the surplus would be required to meet the theoretical model of 1:1 because only 70% of the infiltrate returns to the intravascular space).
A suitable postoperative period should be hemodynamically stable and efficient; blood pressure is one of the considered parameters because postoperative hypotension may be a late sign of the development of shock or a transient consequence of peripheral vasodilation, which is frequent during and after epidural anesthesia.
Use of blood and blood products
With a blood loss of less than 20% of the blood volume (calculation: blood volume is 65 mL/kg in women and 70 mL/kg in men, and blood loss in the superwet technique 1:1 corresponds to 1% to 2% of the aspirate), hemoglobin level of not less than 9 g/dL, and a globular volume of not less than 30 (this parameter has a limited validity because it reflects electrolyte loss only at a later stage), the need to use blood after a liposuction is unlikely.
The decision for blood transfusion is still controversial because the indication based on hematocrit and hemoglobin levels below 30% and 10 g/dL, respectively, has been increasingly disused because of the risk of transmission of diseases and the reduction of costs31. The indication for blood transfusion should consider the balance between the potential risks and the need for replacement and requires medical expertise because these indications are not limited to any single criterion but rely on the assessment of the clinical status of the patient regarding the deficit of oxygen supply32.
In healthy patients, the increase in heart rate or systolic volume may be sufficient to balance the oxygen supply. However, in less favorable conditions, there may be a greater dependence on the arterial oxygen concentration because of the increased oxygen consumption (VO2) for the maintenance of aerobic cellular metabolism, leading to hyperlactatemia33.
An adequate intravascular volume promotes an improvement in perioperative outcomes; however, the amount and composition of fluids vary. Both hypovolemia and hypervolemia can compromise tissue perfusion; thus, a balanced replacement is critical. The intravascular volume may change during neuraxial anesthesia owing to sympathetic block, leading to an increased venous capacitance; use of anesthetic drugs that cause vasodilation; bleeding; dilutional coagulopathy, and hypothermia during surgery34.
The determination of the ideal volume in cases of abrupt hemodynamic changes is always a challenge. Hemodynamic monitoring, laboratory parameters, and clinical evaluation should be the foundations of the recovery and maintenance of adequate hemodynamics. The following criteria can be used as parameters to guide fluid therapy: heart rate, blood pressure, central venous pressure, peripheral oxygen saturation, and urinary flow.
However, these parameters do not indicate the subclinical presence of hypovolemia or hypervolemia. Dynamic parameters, such as variations in the systolic or pulse pressure, plethysmographic waveform of pulse oximetry, monitoring of arterial waves, and calculation of the systolic volume by transesophageal echocardiography can also be used.
Respiratory variations may also provide useful information for intravascular fluid replacement considering that during inspiration in mechanical ventilation, there is an increase in the chest pressure and reduction of the right and left ventricular filling. Therefore, variations in systolic volume due to volume changes can be measured and are responsive to fluid therapy when greater than 10% to 15%34.
The benefits of blood transfusion include the improvement of hemostasis, oxygen transport capacity, and cardiac output owing to the increase in blood volume. A unit of blood will increase the hematocrit by 3%. According to the American Society of Anesthesiologists, the indication of transfusion of packed red blood cells should not be based solely on the hemoglobin levels but rather on the perception of the patient's risk of developing complications related to inadequate oxygenation. However, according to this Society, some considerations should be made regarding transfusion of packed red blood cells:
• A hemoglobin level lower than 6 g/dL, even in healthy and young patients, requires transfusion in cases of acute anemia;
• When the hemoglobin level is higher than 10 g/dL, there is no need for transfusion;
• When the hemoglobin level ranges from 6 to 10 g/dL, red blood cell transfusion should be considered in cases in which there is organ ischemia, potential or current bleeding, or risk factors that trigger complications due to inadequate oxygenation and hypovolemia.
In healthy adults, the adequate intravascular volume provided by volume replacement in cases of bleeding with a 10% decrease in hematocrit level may not decompensate the DO2 because of the increase in cardiac output and oxygen extraction rate and deviation of the oxygen-hemoglobin dissociation curve to the right.
The target hemoglobin level for postoperative transfusions is not well established. However, it is known that levels between 7.1 and 8 g/dL determine a mortality rate of 0.9%, whereas hemoglobin levels from 5.1 to 7.0 g/dL determine a mortality rate of 9.2% in up to 30 days32.
The use of noninvasive real-time monitoring of hemoglobin, although practical, is limited by the lack of documentation and legal responsibility for the examination; thus, this procedure needs to be performed by biochemists to support the indication of transfusion and the awareness regarding the potential risks and benefits.
Blood transfusion may cause ischemia because of the potential occurrence of pro-inflammatory effects (accumulation of pro-inflammatory cytokines) and changes in stored blood31. Stored red blood cells are poor in 2,3 diphosphoglycerate and have a lower capacity to carry oxygen. The stored blood loses nitric oxide activity, limiting the vasodilatory response to hypoxia. Moreover, capillary aggregation and occlusion may occur because of the lower deformability of stored red blood cells. For these reasons, blood transfusions may increase the saturation of mixed venous blood and decrease the oxygen supply to the tissues.
We should consider autologous transfusion (collection and reinfusion of the patient's blood) as a lower-risk option in the case of extensive elective surgery associated with extensive liposuction. In these cases, patients pre-donate blood and receive iron supplementation until the day of surgery.
Another possibility is intraoperative normovolemic hemodilution: Immediately before surgery, the patient's blood is collected in a special bag containing anticoagulants and is kept under adequate cooling. During surgery, the patient is hemodiluted with Ringer's or saline solution. At the end of the surgery up to 5 hours after blood collection, the patient receives his blood, which has a high oxygen transport capacity because of the high hematocrit level and a large capacity of coagulation by the platelets and other coagulation factors.
In the experience of the anesthesiologist author, this practice has been well tolerated by the patients, leading to an improvement in diuresis, lower rate of dizziness in the postoperative period, improved general status, and faster recovery. Normovolemic hemodilution is performed by the anesthesiologist author in medium and extensive liposuctions at the limit of 7% of body weight. The vascular surgeon author also reports that the probability of occurrence of hypercoagulability and DVT is usually lower when normovolemic hemodilution is employed35,36.
Because of the minimal blood loss (1%-2%) during the superwet technique, routine transfusions of blood and blood derivatives should be considered only in extensive liposuction surgeries together with other surgeries or in large liposuctions in patients with clinically poor hemodynamic responses.
Prevention and initial diagnosis of thromboses
I. General protocol for all healthy patients subjected to liposuction and without personal or family history of thrombosis. Patients who are overweight, smokers, users of contraceptive pills, patients under hormone replacement therapy (suspended at least for 15 days before surgery), are included here.
a) Heparin - one of the two options below should be considered:
Unfractionated heparin (UFH) - In the United States, it is common to initiate UFH therapy 6 to 12 hours after surgery, and this regimen has been standardized in clinical trials and is approved by the FDA37. The dose used for the prophylaxis of DVT is 5,000 U of UFH administered subcutaneously. The use of this UFH should be initiated 6 hours after the beginning of surgery and administered every 8 or 12 hours until the patient can ambulate.
Low molecular weight heparin (LMWH) - In Brazil, the most commonly used LMWH is sodium enoxaparin. A single 40-mg dose of enoxaparin sodium should be administered 6 hours after spinal block or 8 hours after withdrawal of the spinal catheter (if used). Enoxaparin should not be used before these time intervals because of the risk of intraspinal hematoma and its severe neurological complications. In case of general anesthesia, enoxaparin may be used immediately after the end of liposuction. LMWH is as effective as UFH in the prophylaxis of DVT but causes less heparin-induced thrombocytopenia37.
Period of maintenance of drug prophylaxis: The need to maintain prophylaxis after hospital discharge is controversial. In orthopedic patients, studies recommend the maintenance of prophylaxis for at least 25 to 30 days after hospital discharge38. However, another study found no clinical justification for such a use39. Prophylaxis should be maintained when the patient remains bedridden or immobilized at home or in the presence of risk factors, such as neoplasias or antitumor therapies.
b) Intermittent pneumatic external compression boots: These are as effective as LMWH and UFH in the prophylaxis of DVT, with the advantage of not increasing the risk of bleeding. They should be installed immediately after anesthetic induction and used until the patient can ambulate normally. In patients with severe obesity, in whom intermittent pneumatic compression boots may not be effective because of the large diameter of the calves, intermittent pneumatic compression devices should be used on the feet. These devices also increase the venous blood flow in the lower limbs and contribute to the effective prophylaxis of DVT40. The use of intermittent pneumatic compression boots has a synergistic effect with the use of LMWH or UFH, consequently decreasing the incidence of DVT further37.
c) Elastic stockings: These are effective in the prevention of DVT, especially when used in conjunction with other methods (UFH or LMWH).
d) Other METHODS: We emphasize that rest in the postoperative period is not advised and that patients should ambulate normally in the morning of the day after liposuction and restrict rest to the usual nocturnal period. We also recommend that the patient make 10 deep hourly inspirations in the immediate postoperative period as an empirical prophylaxis for pulmonary atelectasis.
II. Protocol for patients considered to be at a high risk for developing DVT: patients with a history of DVT or PTE, morbid obesity (BMI >59 kg/m2), inflammatory bowel diseases, and acquired or hereditary thrombophilia (protein C or protein S deficiency, Leiden V factor, and antithrombin III deficiency). In these cases, temporary inferior vena cava filters may be used in addition to the methods mentioned above. These filters should be implanted in the infrarenal vena cava in the preoperative period and removed as soon as the patient can ambulate normally42.
III. Basic diagnosis of DVT/PTE43. Any patient presenting with symptoms of DVT or PTE (e.g., calf swelling, cough, abrupt onset of chest pain, and hemoptysis) should be subjected to the following examinations after liposuction:
a) Echo-Doppler ultrasound of the deep venous system of the lower limbs: In the past few years, echo-Doppler was established as the method of choice for the diagnostic confirmation of DVT.
b) D-dimer: Enzyme-linked immunosorbent assay (ELISA) for D-dimer can be used to exclude the diagnosis of DVT and PTE in cases in which the results are unremarkable because of the high sensitivity of ELISA, despite its low specificity. Lensing et al.43 demonstrated that the association of echo-Doppler and D-dimer measurement is an efficient diagnostic strategy, with less than 1% false negative results.
c) Lung angiotomography: It is the method of choice in confirming the diagnosis of PTE to date.
IV. Fat embolism syndrome: It is a rare complication in the fulminant and subacute forms (1 in 77,000 liposuctions)44 but may be commom in the subclinical form. It is characterized by the obstruction of small pulmonary vessels with fat, which upon hydrolysis by lipases, releases fatty acids, which in turn increase the adhesiveness of neutrophils to endothelial cells.
The proteolytic enzymes on the lysosomes of neutrophils attack the endothelium, leading to endothelial rupture and consequently to hemorrhage and edema in the brain, lungs, and other organs45. Its diagnosis is only clinical with the presence of acute respiratory distress syndrome, central nervous system changes, petechiae, and minor criteria (fever, retinal and urinary changes, hematocrit, thrombocytopenia, positive sputum fat finding, increased erythrocyte sedimentation rate, and relevant brain MRI findings)45,46. The treatment is nonspecific, including ventilatory assistance; however, anticoagulants are contraindicated. The mortality rate is 100% in the fulminant form and 20% in the subclinical form (data from Orthopedic reports)46. Prevention involves avoiding hypoxia, shock, and corticoid use (data from Orthopedics reports)45,46.
Associations with other surgeries
The risk of postoperative venous thrombosis is directly correlated with the surgical time, surgical trauma, and surgical standard. For example, gynecological and urological surgeries are more likely to lead to DVT. Other risk factors for DVT include obesity, coagulopathies, previous thrombosis, and advanced age47.
In cases in which there is an absolute need for combined surgeries, we should consider the surgical time (the longer the surgical time, the higher the morbidity rate), the blood loss (the higher the volume loss, the higher the risk of infection and other complications) and the tissue exposure, which is related to the transoperative hypothermia.
Associations of extensive liposuctions with other procedures in plastic surgery or other specialties should also be avoided because of the increased risk of DVT, PTE, and fat embolism.
CONCLUSIONS
1) Indications of liposuction
a) Localized lipodystrophies
b) Coadjuvant in the treatment of obesity in patients who accept the esthetic limits of the procedure and the transitoriness of the results. Use of a specific consent form in this case is required.
c) Abdominal lipodystrophy in selected patients who do not accept the indicated dermolipectomy/lipoabdominoplasty and agree with the palliative and relatively unpredictable nature of the results obtained with isolated liposuction despite the presence of skin flaccidity. Use of a specific consent form in this case is required.
2) Preoperative period
a) Management of medications and lifestyle
Suspend the following therapies 15 days before surgery:
• MAO inhibitor antidepressants
• Anorexigenics
• Estrogens (hormone replacement therapy)
• Antiarrhythmics
Suspend the following therapies at least 7 days before surgery:
• Phytotherapic compounds: gingko biloba, ginseng, and ginger
• Vitamin E
• Antiplatelet agents (ASA): suspend only in cases of concomitance with other surgical procedures with a high likelihood of bleeding.
Suspend the following therapies on the day of surgery:
Oral hypoglycemic agents
Continue the following therapies even on the day of surgery:
• Antihypertensive drugs
• Lipid-lowering drugs
• Bronchodilators (inhaled beta agonists and leukotriene inhibitors)
• Antidepressants other than MAO inhibitors
• Anticonvulsant drugs
• Anti-thyroid drugs
Suspend the following therapies if possible:
• Contraceptive pills (15 days before surgery)
• Smoking (2 months or at least 24 hours before surgery)
b) Examinations:
• Blood count, coagulogram, and fasting blood glucose, creatinine, and beta human chorionic gonadotropin level evaluation
• Cardiological evaluation
• Anesthesiological evaluation
• Evaluation by a vascular surgeon is fundamental in the following cases: patients with a history of DVT/PTE, patients with morbid obesity (BMI >59 kg/m2), patients with acquired or hereditary thrombophilia (protein C or protein S deficiency, Leiden V factor, and antithrombin deficiency III), and carriers of inflammatory bowel diseases.
3) Transoperative period
a) Anesthesia: epidural or general; local anesthesia only in small areas, with an aspirate volume of approximately 200-500 mL, with particular attention to the immediate and late toxicity of lidocaine up to 14 hours after infiltration.
b) Infiltration: saline solution administered at room temperature with one ampule of adrenaline in 500 mL of serum, respecting the maximum dose of 0.07 mg per kg. Example: Five 1-mg ampules for a 70-kg patient.
c) Change in decubitus: Change the patient's position slowly and in stages. Use of alpha-1 adrenergic agonist (e.g., 1 mg of etilefrine, which may be prepared by diluting 1 mL of etilefrine from a 10-mg ampule in 10 mL of saline) intravenously before changing the decubitus may promote greater safety.
d) Maximum volumeto be aspirated: 5% to 7% of body weight considering the entire bottle provides an additional margin of safety.
4) Postoperative period
a) Discharge on the same day: Although subjective and lacking scientific evidence, the experience of the leading author indicates that patients subjected to liposuction with aspirated volumes of up to 4,000 mL may be discharged on the same day in cases in which the following parameters are observed in addition to the assessment of the clinical conditions mentioned in CFM Resolution 1886 of 2008 (spontaneous ambulation, normal urination, normal sensitivity in the perineal region after spinal block, informed companion, etc.): 8 hours of postoperative observation in the clinic or hospital, discharge only in cases of liposuction of up to 5% of body weight using the superwet technique, maximum aspirated volume of 4,000 mL considering all the contents of the bottle, and absence of the use of lidocaine in the transoperative infiltration.
b) Electrolyte replacement: For an aspirate equal to or less than the infiltrate, use 1 mL/kg/hour of saline or Ringer's solution during fasting (usually 3 hours). It can be replaced or supplemented with an isotonic solution if the patient does not have nausea.
For aspirates higher than the infiltrate, the patient should receive that excess volume in Ringer or physiological solution intravenously. In aspirated volumes greater than 4,000 mL, it is convenient to use a bladder catheter overnight in the clinic and maintain diuresis at 1 mL/kg/hour.
c) Parameters used for a safer postoperative period:
Hemoglobin level of at least 9 g/dL
Diuresis of 1 mL/kg/hour
Alert staff for signs of hypovolemic shock from acute anemia:
Fast and weak pulse (110 bpm or more); fast and short breath (30 rpm or more); systolic blood pressure equal to or less than 80 mmHg; pallor (especially of the ocular mucosa); cyanosis; moist and cold skin (especially of the forehead and palms); excessive thirst; cloudy vision; anxiety, confusion, or unconsciousness; expression of anxiety or distant gaze; and total or partial loss of consciousness.
d) Blood and blood products: Transfusions are indicated considering the laboratory and clinical results of the patient in the postoperative period. It is indicated only in exceptional conditions. There is no indication for its routine prophylactic use, even as autotransfusion or volume normodilution under the superwet technique (rigorously 1:1) in the absence of other concomitant surgeries and with a maximum aspirated volume of 5% of body weight.
However, it can be used in liposuctions of 7% of body weight and in combination with other surgeries or considering the patient's clinical status regardless of the aspirated volume or technique used (wet, superwet, or tumescent). In patients without active bleeding and who are hemodynamically compensated, consider the following criteria (Carson and Kleinman)32:
Hemoglobin level of <6 g/dL - Transfusion is always recommended.
Hemoglobin level of 6-7 g/dL - Transfusion is usually indicated.
Hemoglobin level of 7-8 g/dL - Transfusion is recommended in patients with cardiovascular disease or under conditions that do not respond to fluid replacement, including orthostatic hypotension, tachycardia, and myocardial ischemia.
In patients with active bleeding or comorbidities, in which anemia is determinant for clinical worsening, transfusion may be indicated even in patients with hemoglobin levels of 8-10 g/dL18.
In cases of a high likelihood of extensive blood volume loss, autotransfusion or a normovolemic dilution should be scheduled intraoperatively: the patient's blood should be stored in a special bag before surgery (450 g), kept in a cooler with ice, and reinfused up to 5 hours after storage.
e) Prevention of thrombosis in patients with no additional risk of DVT: Use enoxaparin sodium 40 mg subcutaneously 6 hours after epidural anesthesia or 8 hours after spinal catheter removal (immediately after surgery if general anesthesia was used). Intermittent compression boots during and after surgery until discharge from the clinic and compression stockings during and after surgery for 30 days, (except overnight at home) in the postoperative period. Ensure that the elastic stocking is placed correctly to prevent tourniquet compression by folds.
It is essential to inform the patients and their relatives regarding the contraindication of daytime sleeping from the day after liposuction. For the patients who follow this recommendation and avoid the use of high-heeled shoes, the use of enoxaparin can be interrupted in the day after liposuction.
For patients at a high risk of developing DVT (history and/or the factors mentioned in the Discussion), prevention recommendations should be provided on an individual basis considering the evaluation and conduct of the vascular surgeon and the convenience of liposuction in these patients.
f) Associations with other surgeries: Considering the esthetic and elective nature of liposuction, it is advisable to limit the associations to a single concomitant esthetic procedure with a maximum duration of 3 hours and minimal blood loss whenever possible. The association of liposuctions with gynecological, urological, and orthopedic surgeries should be avoided.
ACKNOWLEDGMENTS
The authors are grateful to Lucas Santiago (Positivo University - Curitiba) for his contribution in the organization of the references.
COLLABORATIONS
JWF Analysis and/or interpretation of data; statistical analyses; final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
AM Writing the manuscript or critical review of its contents.
AASR Writing the manuscript or critical review of its contents.
CTM Writing the manuscript or critical review of its contents.
CLF Writing the manuscript or critical review of its contents.
WMI Writing the manuscript or critical review of its contents.
REFERENCES
1. Instituto de Pesquisas Datafolha. Cirurgia Plástica no Brasil-2009. [acesso 2016 Jan 27]. Disponível em: http://www2.cirurgiaplastica.org.br/wp-content/uploads/2012/11/pesquisa2009.pdf
2. Folha de São Paulo. Cirurgias Plásticas em 2011. [acesso 2016 Jan 27]. Disponível em: http://www2.cirurgiaplastica.org.br/materia-folha-de-sao-paulo/
3. ISAPS SURVEY. Highlights of the ISAPS 2013 Statistics on Cosmetic Surgery. [acesso 2016 Jan 27]. Disponível em: http://www2.cirurgiaplastica.org.br/wp-content/uploads/2014/08/ISAPS_quick_facts.pdf
4. Gomes RS. Critérios de Segurança em lipoaspiração. ACM Arq Catarin Med. 2003;32(4):35-46.
5. Grazer FM, de Jong RH. Fatal outcomes from liposuction: census survey of cosmetic surgeons. Plast Reconstr Surg. 2000;105(1):436-46. DOI: http://dx.doi.org/10.1097/00006534-200001000-00070
6. Associação Brasileira para o Estudo da Obesidade e da Síndrome Metabólica. Mapa da Obesidade. [acesso 2016 Jan 27]. Disponível em: http://www.abeso.org.br/atitude-saudavel/mapa-obesidade
7. Brasil. Ministério da Saúde. VIGITEL 2014. Excesso de Peso e Obesidade. [acesso 2016 Jan 27]. Disponível em: http://portalsaude.saude.gov.br/images/pdf/2015/abril/15/PPT-Vigitel-2014-.pdf
8. Haeck PC, Swanson JA, Gutowski KA, Basu CB, Wandel AG, Damitz LA, et al.; ASPS Patient Safety Committee. Evidence-based patient safety advisory: liposuction. Plast Reconstr Surg. 2009;124(4 Suppl):28S-44S. DOI: http://dx.doi.org/10.1097/PRS.0b013e3181b52fcd
9. Chow I, Alghoul MS, Khavanin N, Hanwright PJ, Mayer KE, Hume KM, et al. Is There a Safe Lipoaspirate Volume? A Risk Assessment Model of Liposuction Volume as a Function of Body Mass Index. Plast Reconstr Surg. 2015;136(3):474-83. DOI: http://dx.doi.org/10.1097/PRS.0000000000001498
10. Illouz YG. Complications of liposuction. Clin Plast Surg. 2006;33(1):129-63. DOI: http://dx.doi.org/10.1016/j.cps.2005.10.001
11. Brasil. Conselho Federal de Medicina. Resolução No 1.711, de 10 de dezembro de 2003. Estabelece parâmetros de segurança que devem ser observados nas cirurgias de lipoaspiração, visando garantir ao paciente o direito de decisão pós-informada e aos médicos, os limites e critérios de execução. [acesso 2016 Jan 27]. Disponível em: http://www.portalmedico.org.br/resolucoes/cfm/2003/1711_2003.htm
12. Sociedade Brasileira de Cirurgia Plástica. Levantamento sobre Lipoaspiração em 2014. [acesso 2016 Jan 27]. Disponível em: http://www2.cirurgiaplastica.org.br/lipoaspiracao-2
13. Giese SY, Bulan EJ, Commons GW, Spear SL, Yanovski JA. Improvements in cardiovascular risk profile with large-volume liposuction: a pilot study. Plast Reconstr Surg. 2001;108(2):510-9. DOI: http://dx.doi.org/10.1097/00006534-200108000-00035
14. Pintarelli G, Gomes RS, Rocha JD. Lipoaspiração: atualização dos fatores de riscos metabólicos e sua importância clínico-cirúrgica. Rev Bras Cir Plást. 2014:29(3):457-67.
15. de Jong RH. Body mass index: risk predictor for cosmetic day surgery. Plast Reconstr Surg. 2001;108(2):556-61. DOI: http://dx.doi.org/10.1097/00006534-200108000-00044
16. Magarão RVQ, Marques AC, Feitosa-Filho GS. Aspirina no perioperatório de cirurgias não cardíacas: o dilema entre manter ou suspender. Rev Bras Clin Med. 2011;9(3):218-24.
17. Duke J. Segredos em Anestesiologia. 3a ed. Rio de Janeiro: Di Livros; 2009.
18. Cavalcanti IL, Cantinho FA, Assad AR. Anestesia para Cirurgia Plástica. Rio de Janeiro: SAERJ; 2005.
19. White PF. Perioperative Drug Manual. 2nd ed. Philadelphia: Saunders; 2004.
20. Assad AR, Volquind D, Vianna PTG, Duarte NM, Pires OC. Educação Continuada em Anestesiologia. Volume I. Rio de Janeiro: Sociedade Brasileira de Anestesiologia; 2011.
21. Mansano AM. Avaliação pré-anestésica. Curso de Educação Continuada em Anestesiologia. Volume I. Rio de Janeiro: SAERJ; 2011. p. 25-42.
22. Correia ACC, Silva PCB, Silva BA. Hipertermia maligna: aspectos moleculares e clínicos. Rev Bras Anestesiol. 2012;62(6):828-37. DOI: http://dx.doi.org/10.1590/S0034-70942012000600007
23. Stoelting RK, Miller RD. Bases de Anestesia. 4ª ed. São Paulo: Roca; 2004.
24. Delfino J, Vale N. Anestesia Peridural, Atualização e Perspectiva. Rio de Janeiro: Atheneu; 2001. DOI: http://dx.doi.org/10.1590/S0034-70942001000600002
25. Manica J. Anestesiologia, Princípio e Técnicas. 2a ed. Porto Alegre: Artmed; 1997.
26. Barash PG, Cullen BF, Stoelting RK, Calahan MK, Stock MC, Ortega R. Manual de Anestesiologia Clínica. 4ª ed. Porto Alegre: Artmed; 2004.
27. Llanos S, Dagnino B, Ponce D, Bonacic S, Navarrete L, Navarrete S, et al. Effect of subcutaneous lidocaine infiltration on blood loss secondary to corporal lipoaspiration: a prospective, randomized, double-masked clinical trial. Aesthetic Plast Surg. 2009;33(5):738-42. PMID: 19484178 DOI: http://dx.doi.org/10.1007/s00266-009-9343-y
28. Brasil. Conselho Federal de Medicina. Resolução CFM No 1.886/2008 de 21 de novembro de 2008. Dispõe sobre as "Normas Mínimas para o Funcionamento de consultórios médicos e dos complexos cirúrgicos para procedimentos com internação de curta permanência". [acesso 2016 Jul 18]. Disponível em: http://www.portalmedico.org.br/resolucoes/CFM/2008/1886_2008.htm
29. Cangiani L. Anestesia Ambulatorial: Recuperação e Critérios de Alta. Curso de Educação à Distância em Anestesiologia. Volume VII. 2007. p. 63-77.
30. Fernandes JW. Lipoaspiração. In: Fernandes JW, ed. Cirurgia Plástica Bases e Refinamentos. Curitiba: Primax; 2012. p. 177-86.
31. Fernandes CR, Ortenzi AV, Mendes FF. Transfusão Sanguínea: Recomendações Atuais e Desfechos Em Longo Prazo Em Diferentes Cenários Clínicos. "Curso de Educação a Distância em Anestesiologia". Rio de Janeiro: SAERJ; 2009. p.103-13.
32. Carson JL, Kleinman S. Indications and hemoglobin thresholds for red blood cell transfusion in the adult. UpToDate. 2017. [acesso 2017 Mar 30]. Disponível em: https://www.uptodate.com/contents/indications-and-hemoglobin-thresholds-for-red-blood-cell-transfusion-in-the-adult
33. Manaker S, Rosen IM. Oxygen delivery and consumption. UpToDate. 2017. [acesso 2017 Mar 30]. Disponível em: https://www.uptodate.com/contents/oxygen-delivery-and-consumption
34. Joshi GP. Intraoperative fluid management. UpToDate. 2017. [acesso 2017 Mar 30]. Disponível em: https://www.uptodate.com/contents/intraoperative-fluid-management
35. Almeida MF. Preoperative normovolemic hemodilution in aesthetic plastic surgery. Aesthetic Plast Surg. 1999;23(6):445-9. DOI: http://dx.doi.org/10.1007/s002669900318
36. Murray D. Acute normovolemic hemodilution. Eur Spine J. 2004;Suppl 1:S72-5. DOI: http://dx.doi.org/10.1007/s00586-004-0755-8
37. Agnelli G, Sonaglia F. Prevention of venous thromboembolism. Thromb Res. 2000;97(1):V49-62. PMID: 10668808 DOI: http://dx.doi.org/10.1016/S0049-3848(99)00207-8
38. Pineo GF, Hull RD. Prophylaxis of venous thromboembolism following orthopedic surgery: mechanical and pharmacological approaches and the need for extended prophylaxis. Thromb Haemost. 1999;82(2):918-24. PMID: 10605804
39. Leclerc JR, Gent M, Hirsh J, Geerts WH, Ginsberg JS. The incidence of symptomatic venous thromboembolism during and after prophylaxis with enoxaparin: a multi-institutional cohort study of patients who underwent hip or knee arthroplasty. Canadian Collaborative Group. Arch Intern Med. 1998;158(8):873-8. PMID: 9570173
40. Morris RJ, Woodcock JP. Evidence-based compression: prevention of stasis and deep vein thrombosis. Ann Surg. 2004;239(2):162-71. PMID: 14745323 DOI: http://dx.doi.org/10.1097/01.sla.0000109149.77194.6c
41. Sachdeva A, Dalton M, Amaragiri SV, Lees T. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev. 2010;(7):CD001484.
42. Kaufman JA, Kinney TB, Streiff MB, Sing RF, Proctor MC, Becker D, et al. Guidelines for the Use of Retrievable and Convertible Vena Cava Filters: Report from the Society of Interventional Radiology Multidisciplinary Consensus Conference. World J Surg. 2007;31(2):251-64. DOI: http://dx.doi.org/10.1007/s00268-006-0292-1
43. Lensing AW, Prandoni P, Prins MH, Büller HR. Deep-vein thrombosis. Lancet. 1999;353(9151):479-85. PMID: 9989735 DOI: http://dx.doi.org/10.1016/S0140-6736(98)04298-6
44. Rees TD, La Trenta GS. Suction-Assisted Lipectomy. In: Aesthetic Plastic Surgery. 2nd ed. Philadelphia: WB. Saunders; 1994.
45. Filomeno LTB, Carelli CR, Silva NCLF, Barros Filho TEP, Amatuzzi MM. Embolia gordurosa: uma revisão para a prática ortopédica atual. Acta Ortop Bras. 2005;13(4):196-208. DOI: http://dx.doi.org/10.1590/S1413-78522005000400010
46. Nogueira FVM, Coelho GVBF, Silveira Junior VF, Andrade CZN, Hetens CMC, Farina Junior JA. Lipoaspiração e embolia gordurosa: Revisão de Literatura. Rev Bras Cir Plást. 2015;30(2):291-4.
47. Pai M, Douketis JD. Prevention of venous thromboembolic disease in surgical patient. UpToDated. 2017. [acesso 2017 Mar 30]. Disponível em: https://www.uptodate.com/contents/prevention-of-venous-thromboembolic-disease-in-surgical-patients
1. Sociedade Brasileira de Cirurgia Plástica, Curitiba, PR, Brazil
2. Universidade Positivo, Curitiba, PR, Brazil
3. Sociedade Brasileira de Anestesiologia, Rio de Janeiro, RJ, Brazil
4. Sociedade Brasileira de Angiologia e Cirurgia Vascular, São Paulo, SP, Brazil
5. Faculdade Evangélica de Medicina do Paraná, Curitiba, PR, Brazil
6. Universidade Federal do Paraná, Curitiba, PR, Brazil
Institution: Clínica Julio Wilson Fernandes, Curitiba, PR, Brazil.
Corresponding author:
Julio Wilson Fernandes
Av. Getulio Vargas
Curitiba, PR, Brazil - Zip Code 80250-180
E-mail: cirurgiaplasticajwf@uol.com.br
Article received: July 24, 2017.
Article accepted: August 7, 2017.
Conflicts of interest: none.




 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter