

Case Report - Year 2017 - Volume 32 -
Pseudoangiomatous stromal hyperplasia: rare case in an 11-year-old girl
Hiperplasia estromal pseudoangiomatosa: caso raro em menina de 11 anos
ABSTRACT
Pseudoangiomatous stromal hyperplasia is a benign disease characterized by excessive proliferation of fibroblasts and myofibroblasts, which can lead to significant breast growth. The presentation is rare, especially among young women and cases requiring mastectomy. This report describes a rare case of an 11-year-old female patient with rapidly progressing mammary hypertrophy, who needed mastectomy and then mammoplasty for complete social integration.
Keywords: Breast; Mammoplasty; Mastectomy; Child
RESUMO
A Hiperplasia Estromal Pseudoangiomatosa (PASH) é uma doença benigna caracterizada pela proliferação excessiva de fibroblastos e miofibroblastos, podendo levar a um crescimento mamário importante. A apresentação é rara, em especial ocasionando necessidade de mastectomia em pacientes jovens. O estudo apresentou o relato de caso raro de uma paciente de 11 anos de idade, com hipertrofia mamária de rápida progressão, com necessidade de mastectomia e posteriormente mamoplastia de aumento para completa reinserção social.
Palavras-chave: Mama; Mamoplastia; Mastectomia; Criança.
Pseudoangiomatous stromal hyperplasia (PASH) of the breasts was first described by Vuitch (1986) as a benign stromal breast lesion, characterized histologically by the proliferation of mature fibroblasts and myofibroblasts with formation of slit-like lesions that simulate vascular channels1. The exact mechanism of tissue proliferation is unknown, but there is evidence to suggest it is related to an excessive response of myofibroblasts to endogenous or exogenous hormonal stimulation, particularly progesterone1-5.
Clinical presentation varies from focal microscopic findings in up to 6.4% of breast biopsies suspected of malignancy; findings may also include an obvious nodule on clinical and mammographic examination2,6,7. A tumor, with rapid growth of the breasts, is rare. PASH mainly affects premenopausal and postmenopausal women using hormone replacement therapy; there is no association with breast cancer.
In most cases, patients are cured by undergoing nodulectomy with wide margins. However, the recurrence rate of PASH is 7-22% of cases4. There are few cases of nodular PASH described in the literature, with only four reports of patients younger than 18 years of age8,9. The youngest patient requiring mastectomy after recurrence was 12 years old9.
Here we describe the case of an 11-year-old girl with giant PASH and the therapeutic approach taken.
CASE REPORT
The patient was a girl aged 11 years and 6 months, with a history of excessive breast growth in the previous 3 months. Prior to the beginning of the complaint, the child had normal pubertal evolution, with poorly developed breast buds. She reported pain in the breasts and in the region of the cervical and thoracic spine, and used her upper limbs to support her breasts. The patient was 4 months post-menarche. She had no personal history or family history of breast cancer.
Examination of the breasts revealed anomalous, symmetrical growth; the presence of large, engorged venous vessels; and increased diameter of the nipple-areolar complex. A marked thoracic kyphosis was observed owing to the excessive weight of the breasts. On palpation, the breasts had increased homogeneous density, with no palpable nodules and no axillary lymph node enlargement (Figure 1). Breast ultrasound and biochemical and hormonal profiles were within normal limits. The patient was assessed by the mastology team and released for surgery.
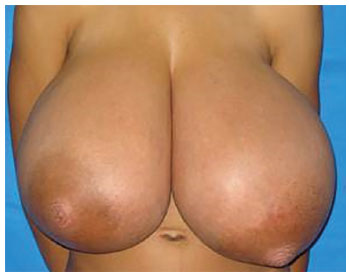
Figure 1. An 11-year-old patient with homogeneously enlarged breasts, with no palpable nodules and no axillary lymph node enlargement.
Initially, the surgical program proposed for the patient was to perform reductive mammoplasty. However, during the intraoperative period, it was observed that the breast tissue presented an anomalous, diffusely infiltrated, grayish-white and fibroelastic aspect, without cleavage planes. It was decided to perform complete excision of the mammary parenchyma and the adjacent skin through an inverted "T" incision, with grafting of the nipple-areolar complex. A vacuum drain was placed subcutaneously bilaterally.
The patient presented a good evolution, with removal of the Brown dressing and vacuum drainage on the fourth postoperative day and total graft integration. The aesthetic results of the scar were good and the patient was satisfied. There was complete improvement of pain symptoms in the breasts and dorsal region, in addition to improvement in the patient's quality of life.
Tissue samples were submitted for frozen section in the operating room, with no evidence of malignancy observed and presenting only hypertrophy with hyperplasia. The pathological study confirmed the absence of malignancy, with the right breast weighing 2820 g and the left breast 3050 g. An immunohistochemical study was performed that showed a profile compatible with bilateral PASH.
After the third post-surgical year, and full pubertal development, the patient began to complain of the lack of breast tissue, this time no longer compatible with her age, leading again to poor quality of life and a desire for breast enlargement (Figure 2).
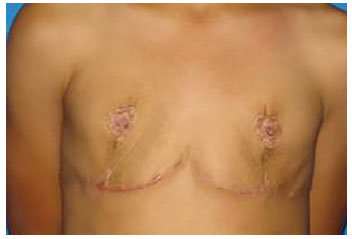
Figure 2. Aspect after mastectomy.
The option was for breast augmentation with placement of breast prostheses. An inframammary incision was made, with submuscular placement of 235 ml high profile round breast implants. The patient presented good postoperative evolution, without intercurrences, and was very satisfied in the immediate and 6-month postoperative periods (Figures 3 and 4).
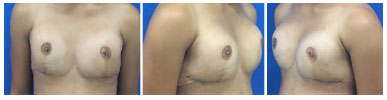
Figure 3. Post-operative aspect 2 weeks after mammoplasty.
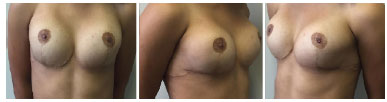
Figure 4. Post-operative aspect 6 months after mammoplasty.
The legal guardian of the patient signed an informed consent form, agreeing to participation in this study.
DISCUSSION
Although there is no definite cause for PASH, the proliferative response of myofibroblasts to endogenous and exogenous hormones seems to represent an important factor in the pathology because it is most commonly diagnosed in premenopausal young women or postmenopausal women using hormone replacement therapy1-4. These patients usually present a palpable and painless nodule that simulates fibroadenoma10,11.
To date, the diagnosis of PASH is made only by excisional surgical biopsy. Fine needle aspiration biopsy usually fails to provide sufficient material to be analyzed for a diagnosis1,2,12.
Local excision with wide margins is the recommended treatment5. However, in cases with diffuse involvement of the parenchyma or recurrent disease, a mastectomy may be necessary3. PASH is not considered a pre-malignant lesion and has an excellent prognosis9. Rapidly growing masses in the breasts of adolescents should raise suspicion of PASH and differential diagnosis be made with low-grade angiosarcoma, phyllodes tumor, and fibroadenoma2,6. In the present case, at the time of surgery there was no possibility of partial resection as any attempt to incise the breast tissue caused intense bleeding.
A presentation with diffuse and rapid breast growth is rare. Few cases have been reported in patients younger than 18 years, especially cases of giant PASH where bilateral mastectomy is required. In this case, non-immediate repair with implants and expanders was justified owing to the age of the child (11 years) to avoid psychosocial discomfort as girls at this age do not usually have significant breast development.
CONCLUSION
Giant pseudoangiomatous stromal hyperplasia should be considered in adolescents who exhibit rapid breast growth. Margin nodulectomy is the treatment of choice in the tumoral form of the lesion, but there are cases in which mastectomy is required. In the case presented, mastectomy and late mammary reconstruction with implants in a 5-year follow-up were effective therapeutic modalities for the healing and rehabilitation of a young patient with giant diffuse pseudoangiomatous breast hyperplasia.
COLLABORATIONS
RAT Writing the manuscript or critical review of its contents.
MSN Final approval of the manuscript; conception and design of the study.
ALC Completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
MMAS Completion of surgeries and/or experiments.
HRP Writing the manuscript or critical review of its contents.
LMF Final approval of the manuscript.
REFERENCES
1. Vuitch MF, Rosen PP, Erlandson RA. Pseudoangiomatous hyperplasia of mammary stroma. Hum Pathol. 1986;17(2):185-91. PMID: 3949338 DOI: http://dx.doi.org/10.1016/S0046-8177(86)80292-1
2. Ibrahim RE, Sciotto CG, Weidner N. Pseudoangiomatous hyperplasia of mammary stroma. Some observations regarding its clinicopathologic spectrum. Cancer. 1989;63(6):1154-60. PMID: 2917318 DOI: http://dx.doi.org/10.1002/1097-0142(19890315)63:6<1154::AID-CNCR2820630619>3.0.CO;2-Q
3. Powell CM, Cranor ML, Rosen PP. Pseudoangiomatous stromal hyperplasia (PASH). A mammary stromal tumor with myofibroblastic differentiation. Am J Surg Pathol. 1995;19(3):270-7. PMID: 7872425 DOI: http://dx.doi.org/10.1097/00000478-199503000-00004
4. Lee JS, Oh HS, Min KW. Mammary pseudoangiomatous stromal hyperplasia presenting as an axillary mass. Breast. 2005;14(1):61-4. DOI: http://dx.doi.org/10.1016/j.breast.2004.09.013
5. Rosen PP. Benign mesenchymal neoplasms. In: Rosen PP, ed. Rosen's Breast Pathology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2009. p. 829-901.
6. Salemis NS. Giant tumoural pseudoangiomatous stromal hyperplasia of the breast in the adolescence. ANZ J Surg. 2011;81(6):469-70. DOI: http://dx.doi.org/10.1111/j.1445-2197.2011.05774.x
7. Degnim AC, Frost MH, Radisky DC, Anderson SS, Vierkant RA, Boughey JC, et al. Pseudoangiomatous stromal hyperplasia and breast cancer risk. Ann Surg Oncol. 2010;17(12):3269-77. DOI: http://dx.doi.org/10.1245/s10434-010-1170-5
8. Gow KW, Mayfield JK, Lloyd D, Shehata BM. Pseudoangiomatous stromal hyperplasia of the breast in two adolescent females. Am Surg. 2004;70(7):605-8. PMID: 15279183
9. Singh KA, Lewis MM, Runge RL, Carlson GW. Pseudoangiomatous stromal hyperplasia. A case for bilateral mastectomy in a 12-year-old girl. Breast J. 2007;13(6):603-6. DOI: http://dx.doi.org/10.1111/j.1524-4741.2007.00499.x
10. Virk RK, Khan A. Pseudoangiomatous stromal hyperplasia: an overview. Arch Pathol Lab Med. 2010;134(7):1070-4. PMID: 20586640
11. Baker M, Chen H, Latchaw L, Memoli V, Ornvold K. Pseudoangiomatous stromal hyperplasia of the breast in a 10-year-old girl. J Pediatr Surg. 2011;46(8):e27-31. DOI: http://dx.doi.org/10.1016/j.jpedsurg.2011.04.063
12. Deniz S, Vardar E, Öztürk R, Zihni İ, Yaǧcı A, Taşlı F. Pseudo-angiomatous stromal hyperplasia of the breast detecting in mammography: case report and review of the literature. Breast Dis. 2014;34(3):117-20. DOI: http://dx.doi.org/10.3233/BD-130360
Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, SP, Brazil
Institution: Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, SP, Brazil.
Corresponding author:
Rafael Alves Tumeh
Rua Borges Lagoa, 512 - Vila Clementino
São Paulo, SP, Brazil Zip Code 04038-000
E-mail: rafaeltumeh@gmail.com
Article received: April 12, 2016.
Article accepted: April 23, 2017.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter