

Review Article - Year 2017 - Volume 32 -
Anatomical analysis of abdominoplasty
Análise anatômica da abdominoplastia
ABSTRACT
Abdominoplasty is a common surgical procedure in plastic surgery. Consequently, several technical variations have been created with the aim of reducing complications and obtaining better results in the abdominal contour. The anatomy of the abdominal region plays an important role in this procedure, since arguments in favor of techniques, such as classic abdominoplasty and lipoabdominoplasty are linked to anatomical principles. This highlights the plastic surgeon's need to have a good knowledge of the anatomy of this region. Not only will this knowledge enable plastic surgeons to understand the reasons for adopting certain surgical procedures, but it will also enable them adapt these procedures to the anatomical characteristics of each patient. In addition, this knowledge will help determine the morphological changes caused by surgery. Therefore, this study aimed to identify studies in the literature focusing on the anatomical description of both classical abdominoplasty and other variants, such as lipoabdominoplasty. It also aimed to discover the anatomical and physiopathological explanations for the major inherent surgical complications.
Keywords: Anatomy; Abdominoplasty; Lipectomy; Postoperative complications.
RESUMO
A abdominoplastia está entre os procedimentos cirúrgicos mais realizados na cirurgia plástica, de forma que muitas variações técnicas têm sido criadas com o objetivo de se reduzirem as complicações e para se obter melhores resultados no contorno abdominal. Nesse contexto, a anatomia da parede abdominal ganha papel de destaque, uma vez que as justificavas em defesa de técnicas, como a abdominoplastia clássica e lipoabdominoplastia, estão atreladas a princípios anatômicos. Isso evidencia a necessidade do domínio da anatomia da região pelo cirurgião plástico, pois, além de entender os motivos de se adotar determinados procedimentos cirúrgicos, o torna mais capacitado de adaptá-los às características anatômicas de cada paciente, além de compreender as alterações morfofuncionais provocadas pela cirurgia. Diante disso, esse trabalho teve o objetivo de buscar na literatura estudos que enfocassem a descrição anatômica da abdominoplastia, tanto clássica quanto de outras variantes, como a lipoabdominoplastia, bem como as explicações anatomofisiopatológicas das principais complicações operatórias inerentes.
Palavras-chave: Anatomia; Abdominoplastia; Lipectomia; Complicações pós-operatórias.
Abdominoplasty is a surgical technique that acts in the tegument by means of abdominal dermolipectomy, in which resection of excess skin and subcutaneous tissue of the lower abdominal region is performed. Moreover, this surgical technique is relevant to the musculoaponeurotic topography, and aims to repair the diastasis of the rectus abdominis aponeurosis.
The development of liposuction has provided new options to achieve the best abdominal contour results. Its combination with abdominoplasty in different operative times, and techniques associating those options at the same surgical time are some alternatives.
The anatomy of the abdominal region is important, since arguments in favor of techniques, such as classic abdominoplasty and lipoabdominoplasty are linked to anatomical principles. Furthermore, serious and/or frequent complications, such as necrosis of the wall, seroma formation, loss or decrease of skin sensitivity of the abdominal wall, etc. have their pathophysiology related to whether or not anatomical limits are considered.
OBJECTIVE
This study aimed to identify studies in the literature focusing on the anatomical description of both classical abdominoplasty and other variants, such as lipoabdominoplasty. It also aimed to find anatomical and physiopathological explanations for the major inherent operative complications.
METHODS
We conducted a review of the literature through searches on PubMed, Medline, Uptodate, and Web of Science database platforms. The following keywords were used: abdominoplasty, lipoabdominoplasty, anatomy of abdominoplasty, anatomy of abdominal wall, vascularization of abdominal wall, arterial vascularization of abdominal wall, necrosis of abdominal wall, abdominoplasty and seroma formation, and innervation of abdominal wall. The references found in the selected articles were used to search for other relevant publications. Studies not establishing a relationship between abdominoplasty and anatomical or physiopathological aspects were excluded. We attempted to prioritize articles published in the last 5 years; however, many studies before this period were cited because they were pioneers, innovators, or even irreplaceable regarding this study's aims.
RESULTS AND DISCUSSION
The results found were grouped and discussed according to the structures of the anterolateral abdominal wall.
Arterial vascularization
Taking into account the vessels nourishing the anterolateral abdominal wall, the arterial supply may be divided into two groups of vessels according to their origin in the abdominal wall: (1) the superolateral division, including the intercostal arteries; subcostal arteries; musculophrenic arteries; and superior epigastric arteries; and (2) the inferior division, including the deep circumflex iliac arteries; lower epigastric arteries, such as branches of the external iliac artery; and superficial epigastric arteries, such as the branch of the femoral artery.
There are also two other arteries arising from the femoral artery: the superficial circumflex iliac arteries and the external superficial pudendal artery1. There are anastomoses between the superficial epigastric artery and the contralateral branch. There is an anastomosis between each superficial artery and the deep epigastric artery2 (Figure 1).
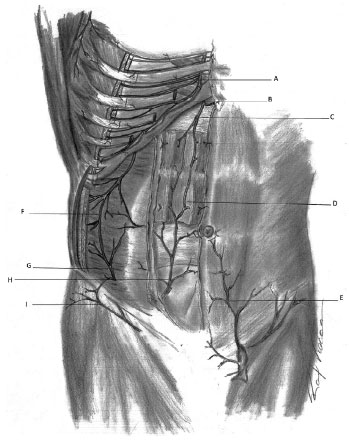
Figure 1. Arterial capillary refill - Schematic representation of the arterial vascularization of the anterior abdominal wall. A: Internal thoracic artery; B: Musculophrenic artery; C: Superior epigastric artery; D: Anastomosis between superior and inferior epigastric arteries; E: Superficial epigastric artery; F: Region of anastomosis between subcostal, lumbar, and deep iliac circumflex arteries; G: Deep iliac circumflex artery; H: Inferior epigastric artery; I: Superficial iliac circumflex artery.
Huger3 divided the anterolateral abdominal wall into three vascular zones. Zone I is the area that extends towards the direction of the cranial pedicle, from the xiphoid process to the pubis. Moreover, it extends in the laterolateral direction, between the lateral margins of the rectus abdominis sheath. Thus, it occupies the middle part of the abdomen. This area is supplied by the superior and lower epigastric arteries.
In the superior area, zone II extends between the anterior superior iliac spines (ASIS). In the lower area, it extends into the inguinal region. Vascularization in this zone is by the superficial and deep arteries. The superficial circumflex iliac arteries, superficial epigastric arteries, and external pudendal arteries represent the superficial ones, while the inferior epigastric arteries in each side represent the deep system in that region.
Zone III, in each side of the body, occupies the region above the ASIS towards the lateral margin of the rectus abdominis sheath, on the border with zone I. Zone III is the region comprising the flanks, hypochondriac regions, and iliac fossa. This zone is supplied by the intercostal, subcostal, and lumbar arteries (Figure 2).
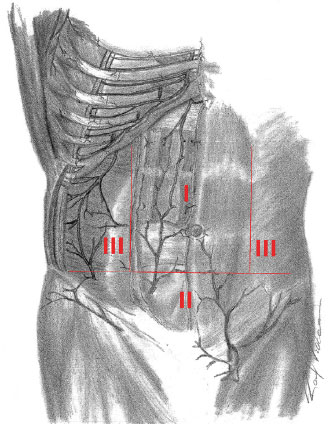
Figure 2. Huger Zones - Schematic representation of areas of vascularization described by Huger.
Analyzing the vascularization of the anterolateral wall with regard to its superficial and deep distribution makes it possible to view the formation of arterial plexuses by the aforementioned vessels. The arterial system that occupies the deep plane is musculoaponeurotic, while the superficial system is subdermal. Numerous vessels make connections between the superficial and deep planes, especially in the navel region. The deep abdominal vessels follow the same arrangement of the abdominal muscles. In the lateral region of the anterolateral abdomen, vessels are arranged in a circumferential, oblique way, while the vessels of the anterior central region display a vertical pattern4.
With regard to the deep plane, vascularization is composed of posterior, subcostal, and lumbar intercostal arteries. This plane is neurovascular, delimited between the internal oblique and transversus abdominis muscles, and forms a vascular network in that lateral region. Moreover, there are anastomoses between the musculophrenic artery and posterior intercostal arteries after deeply and inferolaterally passing by the costal margin.
The deep circumflex iliac artery, which is found in the deep plane of the inguinal region, contributes to the arterial network of the deep neurovascular plane of the abdomen1. This artery runs between the transversus abdominis and the internal oblique muscles, posterior and parallel to the inguinal ligament along the iliac ridge2.
In the central region, the superior and inferior epigastric arteries are arranged longitudinally between the rectus abdominis muscle and the rear end of the sheath, where there are anastomoses in the umbilical region1,4. In addition, there are anastomoses between the terminal branches of the lower intercostal arteries and terminal branches of the superior and inferior epigastric arteries in the lateral region of the abdomen5.
The superior epigastric artery enters the plane between the rectus abdominis muscle and its rear sheath at the level of the seventh costal cartilage through the infrasternal angle. The inferior epigastric artery emerges from the external iliac artery, running in the lateral border of Hesselbach's triangle (inguinal triangle). The vessel then penetrates the plane between the rectus abdominis muscle and its rear sheath, at the level of the linea semilunaris. The anastomosis between the deep epigastric vessels creates a collateral circulation between the external iliac and subclavian arteries2. There are studies showing that anastomosis between the epigastric arteries occurs around the navel. However, a more recent study shows that this occurs, on average, 4.3 cm above the umbilical scar5.
Vascularization of the superficial plane is formed by the following superficial arteries: superficial iliac circumflex artery, superficial epigastric artery, and external pudendal artery. Above all, it is formed by the perforating branches of the deep artery1,4. Dividing the abdomen into four quadrants by the umbilical scar, the lateral regions of the lower quadrants have less vascularization coming from the inferior epigastric arteries. This poses the risk of decreased perfusion of this region if the arteries of the superficial plane are harmed during surgery5.
The perforating branches are myocutaneous, and they pierce the rectus abdominis muscles and their rear sheaths. These branches emerge near the lateral borders, supplying the rectus abdominis muscles, their rear sheaths, the subcutaneous tissue, and skin. More precisely, with regard to location, these perforating arteries are found 2 to 3 cm from the medial edge of the muscles6. On an average, there are six perforating vessels7.
There are perforating arteries of larger and smaller diameters, which create two patterns of arterial supply to the lower abdominal wall. The perforating arteries with smaller diameters mainly supply the deep layer of subcutaneous tissue through the deep arterial plexus. The perforating arteries of larger diameters run to the subdermal plexus, supplying the skin and the more superficial layers of the subcutaneous tissue8.
During classic abdominoplasty, dislocation of the abdominal flap damages the perforating branches of the deep epigastric system in Huger zones I and II. Collateral branches of the superficial circumflex iliac artery of zone II and, especially, the arteries supplying zone III become responsible for the nutrition of the abdominal flap after abdominoplasty.
Consequently, if the displacement of the abdominal flap harms the perforating, lumbar, subcostal, and intercostal vessels, necrosis of the flap becomes a likely complication7,8. Another necrosis situation would occur if these zone III vessels are interrupted by a subcostal scar resulting from a cholecystectomy, which would lead to the interruption of the necrosis of the inferomedial tissues9.
Supraumbilical displacement of the flap aims at providing mobility for the flap, ensuring its lower retraction, and promoting visualization and correction of abdominal diastasis, when it occurs. Liposuction promotes displacement of the supraumbilical flap, thus, increasing its mobility.
Because classic abdominoplasty causes vascular losses in zones I and II, liposuction was adopted as a procedure independent from abdominoplasty. It was performed approximately 6 months later to avoid possible vascular impairment. However, in 1991, Matarasso10 published a study describing liposuction associated with abdominoplasty. In 1995, Matarasso11, in order to ensure that vascular regions would not be impaired, determined safe areas for liposuction when it is performed with abdominoplasty.
In 2003, Saldanha et al.12 described lipoabdominoplasty in order to prevent this displacement from damaging the perforating branches of the deep epigastric system. They introduced the supraumbilical displacement selection restricted to the medial edge of the rectus abdominis, showing that there is a decrease in necrosis.
Many studies have shown that reduced displacement, which occurs in lipoabdominoplasty, preserves most of the perforating vessels. It saves approximately 80% of the blood supply of the abdominal flap compared to classic abdominoplasty13.
A study using Doppler flowmetry in a lipoabdominoplasty with reduced displacement evaluated the periumbilical and right upper quadrant perforator vessels in the pre- and postoperative stages. The study demonstrated that there were, on an average, 5.36 perforating vessels on the right side and 4.92 in the left upper quadrant in the preoperative stage. There were 3.0 on the right side and 3.10 on the left side in the postoperative stage.
This finding confirmed blood preservation of the studied region after abdominoplasty with liposuction in the whole abdomen, and a reduced displacement of the flap. It also showed that liposuction does not damage perforating vessels larger than 1 mm2.
The notable difference between abdominoplasty with liposuction and lipoabdominoplasty is that in lipoabdominoplasty, the whole abdomen may undergo liposuction. This results in discontinuous displacement of the supraumbilical flap, followed by selected displacement for the correction of diastasis and they provide sufficient mobility of the flap and preservation of a great part of the arterial supply, respectively. Conversely, in abdominoplasty with liposuction, only limited areas undergo liposuction. A broad displacement is needed to ensure mobility of the flap at the expense of muscular impairment of zones I and II14.
Venous drainage
The skin and subcutaneous tissue of the abdominal region have a complex subcutaneous venous plexus. Superiorly, this plexus medially drains to a deep vein: the superior epigastric vein, which drains to the internal thoracic vein, which then drains into the subclavian vein. Still superiorly, this plexus drains, laterally, to a superficial vein, the lateral thoracic vein, which is a tributary of the axillary vein. Inferiorly, this plexus drains to a deep vein, the inferior epigastric vein, which then drains into the external iliac vein. It also drains to a superficial vein, the superficial epigastric vein, which empties into the femoral vein15.
In a simplified way, the venous return of the anterolateral wall depends on a thoracoepigastric system, which drains into the axillary, femoral, and deep epigastric system veins4.
Subcutaneous tissue
The subcutaneous tissue may be divided into a superficial and a deep fat layer by the membranous stratum (Scarpa's fascia)4. The superficial layer, also called the areolar layer, has a large amount of fibrous septa4. It has turgid globular cells and a high degree of compaction, resulting in a small interstitial space between them, where small-caliber vessels pass6.
The deep layer, also known as the reticular layer, has few septa, but does not have a well-defined delimitation4. It is composed of loose, smaller globular cells, with a higher fat accumulation and larger intercellular spaces, where higher-caliber vessels pass6 (Figure 3).

Figure 3. Abdominal wall - Schematic representation of the cross-section of the anterior abdominal wall at the infraumbilical level. A: Skin; B: Subcutaneous tissue (areolar layer or superficial adipose tissue or Camper's fascia); C: Superficial fascia (membrane layer or Scarpa's fascia); D: Subcutaneous tissue (deep adipose tissue or subscarpal layer or lamellar layer).
In thin patients, the two layers have similar thicknesses, while, in patients with a high body mass index, the surface layer is much thicker9.
Since the deep layer is generally thinner, with vascularization coming from the perforating arteries, this blood supply tends to be harmed if the flap is displaced. Conversely, because the surface layer tends to be thicker, it plays an important role in thermoregulation. Its blood supply comes from both the perforating vessels and subdermal plexus, which keep it intact, especially in lipoabdominoplasty. The superficial vascularization system is usually not impaired during flap displacement1.
Skin vascularization depends on the subdermal plexus of the superficial fat layer. Therefore, excision of the deep layer of adipose tissue may be performed. However, excision of the superficial layer has numerous restricted areas, since excision of this layer may harm the subdermal plexus also responsible for skin vascularization11. Thus, the superficial layer is protective. The deep layer varies in thickness according to the abdominal region. It should undergo liposuction for satisfactory results10.
Computed tomography analysis of the subcutaneous tissue shows that the umbilical region (analyzing it immediately above the umbilical scar) is approximately 5 mm thicker than the suprapubic region (at the level of the abdominoplasty incision line). Analyzing the superficial and deep layers separately in both regions, the surface layer is thicker than the deep layer. Comparing the deep layer of the two regions, the umbilical region deep layer is approximately 9 mm thicker than the same layer in the suprapubic region.
These findings suggest the need to conduct resection of the deep layer of the abdominal flap, since patients with these differences in thickness tend to have a greater chance of displaying a protrusion above the suture line after abdominoplasty16.
Membrane layer (scarpa's fascia) or superficial fascia
It is shown that preserving Scarpa's fascia has advantages due to several factors. Bleeding is reduced because the inferior perforating vessels are preserved. A homogeneous support for the abdominal flap, which becomes thinner as it is inferiorly retracted, is created; and containment of scar laterally is performed, which provides better adherence between the flap and deep layers13.
In addition, the membranous layer (Scarpa's fascia) has been considered by many authors as the key to closing the abdominoplasty. If the membranous layer is not restructured, reinforcing its continuity by suturing it may cause retraction in the cephalic direction towards the umbilical scar, leading to a transverse suprapubic depression and a depressed scar4,17. Thus, suturing Scarpa's fascia reduces the skin tension generated during abdominoplasty. Moreover, it generates a thinner, better quality scar, avoiding its possible migration1,17.
Preservation and suture of Scarpa's fascia in the infraumbilical region are promoted. Moreover, its suspension is defended superiorly and medially, as well as with a continuous suture fixation in the abdominal wall. This maneuver elevates the lower incision and anterior thigh skin; thus, attenuating the closing tension in the groin area. In addition, despite the need for supporting lymphoscintigraphy studies, this suspension and fixation promote an «opening» of the lymphatic channels in the deep adipose layer, improving the lymphatic drainage of the region18.
Nevertheless, there is a possibility of partial resection of Scarpa's fascia in the central umbilical region limited by the lateral margins of the recti abdominis, with subsequent rapprochement and suture in the midline level of Scarpa's fascia preserved in both sides of the abdomen. Such a technique is justified by the improved waist contour definition it promotes, in addition to the reduced dead space in the midline19.
Zones of adherence
The lower torso has facial annexes called zones of adherence between the skin and muscle fascia coating. These areas promote fixation of the skin to the musculoaponeurotic layer, avoiding elevation or descent of the muscle with changes in weight, aging, and surgical procedures20.
There are five zones of adherence in the torso. Three of them are horizontal, and two are vertical. For the horizontal zones, the first zone is bilaterally located in the inguinal ligament extending to the ASIS; the second is located immediately above the mons pubis; the third is bilaterally located between the hip and the superolateral region of the thigh. For the vertical zones, one zone covers the spine in the posterior midline, while the other zone overlaps the linea alba1,9.
In abdominoplasty, there is an argument suggesting that the suture of Scarpa's fascia should be carried out to avoid displacement of the cephalic scar, and an argument stating the zones of adherence also do this1,9. It is widely believed the suture of Scarpa's fascia also prevents this migration because it facilitates anchoring of zones of adherence1.
Lymphatic drainage
The lymphatic drainage of the anterolateral abdominal wall may be divided into a superficial system, situated in the adipose layer on Scarpa's fascia, and a deep system, located in the adipose layer under the fascia. The deep system drains to the external iliac lymph nodes. The superficial vessels, above the navel, drain to the axillary lymph nodes, while the vessels below the navel drain to the superficial inguinal lymph nodes1,15 (Figure 4).
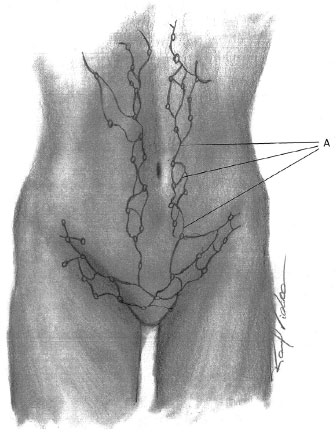
Figure 4. Lymphatic drainage - Schematic representation of the superficial lymphatic drainage of the anterior abdominal wall. A: Superficial lymph vessels.
Generally, the lymphatic drainage in the immediate postoperative stage of classic abdominoplasty is predominantly directed to the axillary lymph nodes. Later, it returns to normal with the restitution of the lymphatic channels to the superficial inguinal lymph nodes4.
Lymphatic Drainage, Scarpa's Fascia, and Seroma Formation
Seroma formation consists of numerous etiological mechanisms. Among these are lesions in blood vessels and lymphatic channels, dead space formation resulting from flap displacement, and the presence of shearing forces between the abdominal flap and superficial fascia. This is caused by the movement of two structures against each other, together with the release of inflammatory mediators7.
Dissection of the abdominal flap made up to the plane of Scarpa's fascia, in the umbilical region, preserves both the fascia and the layer of subfascial fat, along with the deep lymphatic system located in this layer12,13.
A comparative ultrasound analysis between classic abdominoplasty and lipoabdominoplasty indicates a significant incidence of postoperative seroma formation in patients who undergo classic abdominoplasty. This minor seroma formation in patients undergoing lipoabdominoplasty is explained by the generation of a smaller displacement of the supraumbilical flap (forming a smaller dead space) by this technique. Additionally, it fosters a greater preservation of lymphatic drainage, since preservation of Scarpa's fascia maintains the lymph vessels found in subscarpal fat21. In numerical terms, a study showed an 86.7% reduction in the rate of seroma formation if lipoabdominoplasty is employed instead of classic abdominoplasty22.
Not only is seroma formation related to the lack of preservation of Scarpa's fascia and, consequently, the lymph vessels found in the subscarpal layer, seroma formation in classic abdominoplasty is also related to liposuction of the superficial adipose layer of the abdominal flap together with its aggressive displacement. These two procedures leave large portions of the subscarpal adipose tissue partly devitalized. Thus, if the abdominal flap is reinserted, these portions of tissue may lead to decreased adherence. Thus, they may generate spaces likely to accumulate liquid, with a lower capacity for liquid resorption23.
However, reduced seroma formation, resulting from preservation of Scarpa's fascia and subscarpal adipose tissue along with the lymphatic system, is still very questionable. According to some authors, there is no evident physiological basis to affirm that preserving Scarpa's fascia maintains deep lymphatic drainage. They explained that the lymphatic drainage lost in classic abdominoplasty, due to the lack of preservation of Scarpa's fascia and subscarpal adipose tissue, is irrelevant.
Moreover, they stated that if a high incidence of seroma formation occurs in techniques that do not preserve the fascia, the etiology is probably due to the use of electrodessication to elevate the flap. This explanation is based on the grounds that seroma is characterized as an inflammatory exudate, instead of a purely lymphatic collection. Therefore, electrodessication would incite an inflammatory response that increases capillary permeability and fluid accumulation24.
Nevertheless, a recent prospective study compared dissection of the abdominal flap in patients who underwent classic abdominoplasty. In one of the groups, dissection was performed with an electrocautery; in the other group, dissection was performed with a scalpel (the electrocautery was used only for possible hemostasis). The study found no significant difference among the seroma formation rates: in the group subjected to dissection with the electrocautery, the rate was 17.2%, while in the other group, the rate was 20.1%25.
Musculoaponeurotic system
The anterolateral wall of the abdomen is composed of three flat and two vertical muscles. All these muscles are bilateral pairs. The flat muscles are concentric, and are anterolaterally located. These muscles are the external oblique, internal oblique, and transversus abdominis muscles. There are two vertical muscles that are adjacent to the midline: rectus abdominis and the pyramidal muscle. All these muscles and their aponeuroses are externally coated by a coating fascia. The fascia of the abdominal wall that separates the deep face of the transversus abdominis muscle of the extraperitoneal fat is called the endoabdominal fascia or, in this case, since it has a relationship with the transversus abdominis muscle, is called the transversal fascia15.
The lateral borders of the rectus abdominis reflect vertical depressions in the skin called linea semilunaris. These points of reference correspond to the vertical location in which the muscle fibers of the three flat muscles become aponeurotic, and they converge to form the rectus abdominis sheath1,15.
The sheath of the rectus abdominis is divided into anterior and posterior layers. The posterior layer is formed, in the superior three-quarters of the rectus abdominis, by the aponeurosis of the transversus abdominis muscle and the posterior part of the internal oblique muscle aponeurosis, which is divided into anterior and posterior parts in that region. The inferior quarter of the rectus sheath is posteriorly deficient, since the muscle is coated by the transversal fascia.
This posterior zone of the transition of the rectus sheath and the transversal fascia are represented by an inferior aponeurotic horizontal line on the posterior layer of the sheath called the arched line. Above this line, the anterior sheath layer is formed by the anterior division of the internal oblique aponeurosis and the external oblique aponeurosis. Below the line, the anterior layer is formed by three flat muscle aponeuroses15.
On one side, the aponeuroses converge and intertwine with the opposite side on the midline before forming the linea alba, which continues vertically separating the sheaths from the rectum bilaterally. The linea alba is one of the areas of natural weakness in the abdominal wall, and it is prone to diastasis caused by tissue tension, such as in pregnancy or intracavitary distension4. Abdominal diastasis is linked to weakening and separation of the aponeurotic fibers, which converge forming the linea alba, from the midline, with no hernia formation1. Correction of this aponeurotic weakening is one of the functions of abdominoplasty, which repairs it using plication of the rectus abdominis sheath.
One of the functions of the abdominal muscles is the maintenance of supra-atmospheric intra-abdominal pressure. However, after abdominoplasty, an increase in this pressure is common due to plication to correct abdominal diastasis, which may cause complications. Among the complications, there is impairment of pulmonary function, especially in patients with chronic obstructive pulmonary disease.
This occurs because the increased intra-abdominal pressure elevates the diaphragm26. A quantitative and qualitative analysis of plication with pulmonary impairment reveals that its employment to correct diastasis of the rectus abdominis muscles significantly reduces spirometric values. These are assessed on the second postoperative day and tend to normalize around the fifteenth day27.
Not only is there plication of the sheaths of the rectum, there is also plication of the external oblique muscle aponeurosis. This additional plication may be suggested to patients needing full removal of the excess skin and subcutaneous tissue between the umbilicus scar and the pubic region. It may also be suggested to patients who, even after the plication of the rectus abdominis sheath, continue with flaccidity in the lateral and inferior region of the musculoaponeurotic layer. Nonetheless, there is a study showing that this kind of plication does not change the spirometric values. This is possibly due to the layer of loose connective tissue between the internal and external oblique muscles, which enables a slip between both of them27.
Innervation of the anterolateral abdominal wall
Cutaneous and motor innervation of the anterolateral abdominal wall is supplied by the anterior branches of the spinal nerves (from T7 to T12) and the anterior branch of the L1 spinal nerve. The anterior branches of the spinal nerves from T1 to T11 are called intercostals. Intercostal nerves from T7 to T11, after emerging from the intercostal muscles and entering the neurovascular plane between the internal oblique and transversus abdominis muscles, become the thoracoabdominal nerves of the anterior wall of the abdomen. The anterior branch of T12 is the subcostal nerve. The anterior branch of the L1 spinal nerve is divided into two branches, the iliohypogastric and ilioinguinal15 (Figure 5).
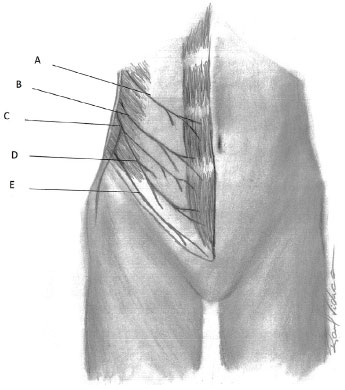
Figure 5. Innervation - Schematic representation of the anterolateral abdominal wall innervation. A: Thoracoabdominal nerve (T10); B: Subcostal nerve (T12); C: Anterior branch of L1 spinal nerve; D: Iliohypogastric nerve (L1); E: Ilioinguinal nerve (L1).
The other nerves emit a lateral cutaneous branch and an anterior cutaneous branch, except for the anterior L1 branch. The lateral cutaneous branches emerge from the anterolateral muscle wall to enter the subcutaneous tissue along the anterior axillary line, while its anterior cutaneous branches pierce the rectus abdominis sheath to enter the subcutaneous tissue at a short distance from the median plane15.
Thus, the cutaneous innervation of the anterolateral abdominal wall is laterally innervated to the clavicular midline by the lateral cutaneous branches, and it is medially innervated to the clavicular midline by the anterior cutaneous branches. These nerves, from T7 to T9, sensitively innervate the skin above the navel. T10 innervates the skin around the navel, and T11 and T12 innervate the skin below the navel15.
The iliohypogastric and ilioinguinal nerves innervate the skin below the navel and other regions. The former innervates the skin on the iliac crest, superior inguinal region, and hypogastrium, while the latter innervates the skin of the lower inguinal region, the anterior part of the scrotum or labia majora, and the adjacent medial side of the thigh15.
An alteration in the arrangement of the abdominal wall nerves occurs after surgery. Dermatomes T5-T12 move inferiorly in the perioperative stage. In addition, dermatomes T10, T11, and T12 are generally resected with the skin. Consequently, dermatomes from T5 to T9 become magnified in the abdominal wall. Dermatome T9 is placed immediately above the incision4,28.
A study suggests that these innervation modifications are of paramount importance if patients undergoing abdominoplasty also undergo new abdominal surgery under spinal anesthesia. The anesthesiologist must know that the lower the abdominal surgery level, the higher the medullary blockade should be28.
Regardless of length, flap displacement generally creates an area of paresthesia, characterized by numbness in the umbilical region in the shape of a triangle. One of its vertices is the umbilical scar, which has a transverse scar resulting from classic abdominoplasty as an opposite base. However, this numbness tends to disappear with time. Its non-disappearance suggests injury to one of the abdominal wall nerves susceptible to impairment during surgery. Among them, the iliohypogastric and ilioinguinal nerves may be affected during plication of the anterior layer of the rectus abdominis9.
The lateral femoral cutaneous nerve may also be affected. It passes by the inguinal ligament either posteriorly or through it, and may be located in a medial, previous or intermediary position in relation to the ASIS4. Generally, it is located 1 to 6 cm medial to the ASIS9. This nerve courses inferiorly in the thigh. It first crosses the insertion of the proximal sartorius muscle, and then, it goes under the fascia lata to a variable point, usually 10 cm below the inguinal ligament, where it perforates the fascia to become subcutaneous. In this region, the nerve divides itself into two branches to supply the anterolateral thigh skin28.
Therefore, a surgeon's special attention is needed, since the extension of the incision of the abdominoplasty may reach the emergence of the nerve topography28. A study suggests that injury to this nerve caused serious damage to an operated patient's sexual function because of sensitive impairment of the region it innervates29.
Comparing lipoabdominoplasty with classic abdominoplasty, regarding innervation, there is a report on the improvement of the former technique in relation to the latter. It is argued that lipoabdominoplasty preserves the innervation responsible for skin sensitivity of the abdominal flap, especially concerning superficial pain and touch if it is stimulated by temperature, vibration, and pressure13.
The umbilical scar and other points of reference
The location of the navel is of considerable importance to ensure its correct positioning after abdominoplasty1. Considering the vertebral level as reference, the umbilical scar is approximately at the level of L3 and L44 (Figure 6). With respect to ASIS, they are located at the same imaginary line level connecting them4. Focusing on the pubic symphysis and the pubic hair line, it is located approximately 14 cm above the first spine and 10 cm from the second one1.
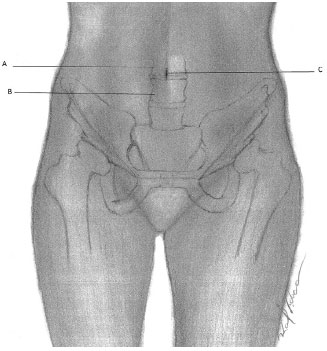
Figure 6. Umbilical Scar - Schematic representation of the relationship between the umbilical scar and lumbar vertebrae. A: L3 Backbone; B: L4 Backbone; C: Umbilical scar.
A study suggested a formula for finding the ideal location of the final position of the umbilical scar without employing these anatomical relationships. The formula is based on a 1/1.5-proportion (or a 1/1,618-proportion by Leonardo da Vinci) between the pubis and the umbilical scar and between the umbilical scar and the xiphoid process. In conclusion, the ideal position of the navel (U) in relation to the pubic symphysis, is two times the distance from the pubic symphysis to the xiphoid process (d) divided by five (U = 2d/5)30.
Another important factor pertaining to the umbilical scar is its arterial supply provided by the subdermal plexus and the inferior epigastric arteries of each side1. The vessels originating from these arteries have an axial arrangement towards the umbilical scar, and have a small caliber, which may impair perfusion. This may lead to necrosis if traction or twisting movements are performed during their preparation and replacement.
Thus, to provide better blood flow to the navel skin, a broad diameter of the line of incision around the umbilicus must exist (at least 2.5 cm). The vessels that supply it, axially distributed toward the navel, do it in various ways5. Moreover, the integrity of its blood supply is also guaranteed by a thick adipose layer maintained close to the navel, since the subdermal plexus is found in it9.
Not only is the umbilical scar an important point of reference, the limit of the lower abdomen (pubic hair line or line of Dumm) is also relevant. According to the authors, on average, a 6-cm distance between the lower limit and the vulvar commissure is needed to avoid any type of distortion4.
CONCLUSION
Anatomical knowledge of the anterolateral abdominal wall is essential to avoid complications during an abdominoplasty. Knowing the anatomy of the region enables a plastic surgeon understand the reasons for adopting certain surgical procedures and adapt to the anatomical characteristics of each patient. This can be coupled with an understanding of possible anatomical changes caused by the surgery.
COLLABORATIONS
FVTB Analysis and/or interpretation of data; statistical analyses; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript.
LETA Final approval of the manuscript; critical review of its contents.
LSB Final approval of the manuscript; critical review of its contents.
RVTB Conception and design of the study; completion of surgeries and/or experiments.
REFERENCES
1. Nahai FR. Anatomic considerations in abdominoplasty. Clin Plast Surg. 2010;37(3):407-14. DOI: http://dx.doi.org/10.1016/j.cps.2010.03.003
2. Graf R, de Araujo LR, Rippel R, Neto LG, Pace DT, Cruz GA. Lipoabdominoplasty: liposuction with reduced undermining and traditional abdominal skin flap resection. Aesthetic Plast Surg. 2006;30(1):1-8. DOI: http://dx.doi.org/10.1007/s00266-004-0084-7
3. Huger WE Jr. The anatomic rationale for abdominal lipectomy. Am Surg. 1979;45(9):612-7. PMID: 159651
4. Jaimovich CA, Mazzarone F, Parra JFN, Pitanguy I. Semiologia da parede abdominal: seu valor no planejamento da abdominoplastia. Rev Soc Bras Cir Plast. 1999;14(3):21-50.
5. O'Dey DM, Heimburg DV, Prescher A, Pallua N. The arterial vascularisation of the abdominal wall with special regard to the umbilicus. Br J Plast Surg. 2004;57(5):392-7. PMID: 15191818 DOI: http://dx.doi.org/10.1016/j.bjps.2004.02.008
6. Matos Júnior WN, Jiménez FVC, Rocha RP. Anatomia descritiva na lipoabdominoplastia. In: XLI Congresso Brasileiro de Cirurgia Plástica; 2004; Florianópolis, SC, Brasil. p. 17-20.
7. Atiyeh BS, Ibrahim A, Hayek S. The Ultimate Art of Sculpture Abdominoplasty. Ann Plast Surg Reconstr Microsurg. 2010;4:75-93.
8. El-Mrakby HH, Milner RH. The vascular anatomy of the lower anterior abdominal wall: a microdissection study on the deep inferior epigastric vessels and the perforator branches. Plast Reconstr Surg. 2002;109(2):539-43. DOI: http://dx.doi.org/10.1097/00006534-200202000-00020
9. Aly AS, Rotemberg SC, Cram A. Abdominoplasty. In: Chung KC, Disa J, Gosain A, Kinney B, Rubin P, eds. Plastic Surgery: Indications and Practice. Philadelphia: Saunders; 2009. p. 1609-1626.
10. Matarasso A. Abdominolipoplasty: a system of classification and treatment for combined abdominoplasty and suction-assisted lipectomy. Aesthetic Plast Surg. 1991;15(2):111-21. PMID: 2035359 DOI: http://dx.doi.org/10.1007/BF02273843
11. Matarasso A. Minimal-access variations in abdominoplasty. Ann Plast Surg. 1995;34(3):255-63. PMID: 7598381 DOI: http://dx.doi.org/10.1097/00000637-199503000-00006
12. Saldanha OR, De Souza Pinto EB, Mattos WN Jr, Pazetti CE, Lopes Bello EM, Rojas Y, et al. Lipoabdominoplasty with selective and safe undermining. Aesthetic Plast Surg. 2003;27(4):322-7. PMID: 15058559 DOI: http://dx.doi.org/10.1007/s00266-003-3016-z
13. Saldanha OR, Azevedo SF, Delboni PS, Saldanha Filho OR, Saldanha CB, Uribe LH. Lipoabdominoplasty: the Saldanha technique. Clin Plast Surg. 2010;37(3):469-81. PMID: 20624545 DOI: http://dx.doi.org/10.1016/j.cps.2010.03.002
14. Levesque AY, Daniels MA, Polynice A. Outpatient lipoabdominoplasty: review of the literature and practical considerations for safe practice. Aesthet Surg J. 2013;33(7):1021-9. DOI: http://dx.doi.org/10.1177/1090820X13503471
15. Moore KL, Dalley AF, Agur AMR. Abdome. In: Moore KL, Dalley AF, Agur AMR. Anatomia Orientada para a Clínica. 6a ed. Rio de Janeiro: Guanabara Koogan; 2012. p.181-323.
16. Harley OJ, Pickford MA. CT analysis of fat distribution superficial and deep to the Scarpa's fascial layer in the mid and lower abdomen. J Plast Reconstr Aesthet Surg. 2013;66(4):525-30. DOI: http://dx.doi.org/10.1016/j.bjps.2012.12.003
17. Bozola AR. Abdominoplasty: same classification and a new treatment concept 20 years later. Aesthetic Plast Surg. 2010;34(2):181-92. DOI: http://dx.doi.org/10.1007/s00266-009-9407-z
18. Wulkan M, Hurwitz D. Preservação e suspensão da fáscia de Scarpa na Abdominoplastia. Rev Bras Cir Plást. 2010;25(3):490-8. DOI: http://dx.doi.org/10.1590/S1983-51752010000300016
19. Mossaad BM, Frame JD. Medial advancement of infraumbilical Scarpa's fascia improves waistline definition in "Brazilian" abdominoplasty. Aesthetic Plast Surg. 2013;37(1):3-10. DOI: http://dx.doi.org/10.1007/s00266-012-0004-1
20. Aly AS. Options in lower truncal surgery. In: Aly AS, ed. Body contouring after massive weight loss. St Louis: Quality Medical; 2006. p.59-70.
21. Di Martino M, Nahas FX, Novo NF, Kimura AK, Ferreira LM. Seroma em lipoabdominoplastia e abdominoplastia: estudo ultrassonográfico comparativo. Rev Bras Cir Plást. 2010;25(4):679-87. DOI: http://dx.doi.org/10.1590/S1983-51752010000400021
22. Costa-Ferreira A, Rebelo M, Silva A, Vásconez LO, Amarante J. Scarpa fascia preservation during abdominoplasty: randomized clinical study of efficacy and safety. Plast Reconstr Surg. 2013;131(3):644-51. PMID: 23446574 DOI: http://dx.doi.org/10.1097/PRS.0b013e31827c704b
23. Neaman KC, Armstrong SD, Baca ME, Albert M, Vander Woude DL, Renucci JD. Outcomes of traditional cosmetic abdominoplasty in a community setting: a retrospective analysis of 1008 patients. Plast Reconstr Surg. 2013;131(3):403e-10e. DOI: http://dx.doi.org/10.1097/PRS.0b013e31827c6fc3
24. Swanson E. Scarpa fascia preservation during abdominoplasty: randomized clinical study of efficacy and safety. Plast Reconstr Surg. 2013;132(5):871e-873e. PMID: 24165647 DOI: http://dx.doi.org/10.1097/PRS.0b013e3182a4c4bc
25. Marsh DJ, Fox A, Grobbelaar AO, Chana JS. Abdominoplasty and seroma: a prospective randomised study comparing scalpel and handheld electrocautery dissection. J Plast Reconstr Aesthet Surg. 2015;68(2):192-6. PMID: 25456290 DOI: http://dx.doi.org/10.1016/j.bjps.2014.10.004
26. Hunter GR, Crapo RO, Broadbent TR, Woolf RM. Pulmonary complications following abdominal lipectomy. Plast Reconstr Surg. 1983;71(6):809-17. PMID: 6856697 DOI: http://dx.doi.org/10.1097/00006534-198306000-00011
27. Rodrigues MA, Nahas FX, Gomes HC, Ferreira LM. Ventilatory function and intra-abdominal pressure in patients who underwent abdominoplasty with plication of the external oblique aponeurosis. Aesthetic Plast Surg. 2013;37(5):993-9. DOI: http://dx.doi.org/10.1007/s00266-013-0158-5
28. da Silveira Carvalho CG, Baroudi R, Keppke EM. Anatomical and technical refinements for abdominoplasty. Aesthetic Plast Surg. 1976;1(1):217-28. DOI: http://dx.doi.org/10.1007/BF01570254
29. Floros C, Davis PK. Complications and long-term results following abdominoplasty: a retrospective study. Br J Plast Surg. 1991;44(3):190-4. PMID: 1827355 DOI: http://dx.doi.org/10.1016/0007-1226(91)90125-4
30. Leão C. Lipodorsoabdominoplastias: 10 anos de experiência. Rev Bras Cir Plást. 2010;25(4):688-94. DOI: http://dx.doi.org/10.1590/S1983-51752010000400022
1. Universidade Federal de Ouro Preto, Ouro Preto, MG, Brazil
2. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
3. Instituto Médico Legal, Belo Horizonte, MG, Brazil
4. Associação Brasileira de Medicina Legal, Ouro Preto, MG, Brazil
5. Universidade de Rio Verde, Aparecida de Goiânia, GO, Brazil
Institution: Universidade Federal de Ouro Preto, Ouro Preto, MG, Brazil.
Corresponding author:
Filipe Vidica Teodoro Barcelos
Rua 1057, Quadra 130, Lote 21 - Setor Pedro Ludovico
Goiânia, GO, Brazil Zip Code 74825-210
E-mail: filipevidica@gmail.com
Article received: March 18, 2015.
Article accepted: February 9, 2017.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter