

Review Article - Year 2016 - Volume 31 -
General treatment and wound management in hereditary epidermolysis bullosa: indication and experience using silver hydrofiber dressing
Tratamento geral e das feridas na epidermólise bolhosa hereditária: indicação e experiência usando curativo de hidrofibra com prata
ABSTRACT
INTRODUCTION: Hereditary epidermolysis bullosa (EB) is a rare disorder characterized by cutaneomucous fragility, with formation of blisters during minimal trauma. Treatment consists of clinical and nutritional support and management of pain and skin lesions. Silver hydrofiber (Aquacel Ag®) is a type of carboxymethylcellulose fiber dressing with silver that can be used in selected cases of EB.
OBJECTIVE: To review the literature on the general treatment and management of cutaneous lesions in congenital EB and evaluate the indication and experience of using silver hydrofiber dressing.
METHODS: The review included original articles and systematic reviews published between 2009 and 2014. We also selected two patients with congenital EB treated at the Plastic Surgery Division of Hospital das Clínicas of the Faculty of Medicine of Ribeirão Preto at the University of São Paulo.
RESULTS: There is a shortage of scientific evidence related to the treatment of skin lesions in congenital EB, with most recommendations being based on expert opinions. Hydrofiber is indicated in most consensuses for wounds with some exudation and has been shown to be more absorbent than alginate. In our experience, there was apparent improved control of pain, bleeding, and hypothermia with the use of hydrofiber, which has the advantage of not requiring daily changes and can remain on the wound for up to two weeks.
CONCLUSIONS: The general and lesion treatments in EB are challenging. Hydrofiber with silver is a treatment option for wounds in hereditary EB, without the need for daily dressing changes.
Keywords: Epidermolysis bullosa; Therapeutics; Wounds and lesions; Occlusive Dressings; Silver compounds.
RESUMO
INTRODUÇÃO: Epidermólise bolhosa (EB) hereditária é uma desordem rara caracterizada pela fragilidade cutaneomucosa, com formação de bolhas ao mínimo trauma. O tratamento consiste em suporte clínico, nutricional, manejo da dor e das lesões cutâneas. A hidrofibra com prata (Aquacel Ag®) é um tipo de curativo de fibra de carboximetilcelulose e prata que pode ser utilizada em casos selecionados de EB.
OBJETIVO: Revisão da literatura sobre o tratamento geral e o manejo das lesões cutâneas na EB congênita, além de avaliar a indicação e experiência usando curativo de hidrofibra com prata.
MÉTODOS: A revisão incluiu artigos originais e revisões sistemáticas, publicados entre 2009 e 2014. Selecionamos ainda dois pacientes com EB congênita tratados na Divisão de Cirurgia Plástica do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo.
RESULTADOS: Há escassez de evidências científicas relacionadas ao tratamento das lesões cutâneas na EB congênita, sendo a maioria das recomendações baseadas em opiniões de especialistas. A hidrofibra está indicada na maioria dos consensos para feridas com alguma exsudação e mostrou-se mais absorvente que o alginato. Em nossa experiência, houve aparente melhor controle da dor, do sangramento e da hipotermia com o uso da hidrofibra, que apresenta a vantagem de não necessitar de trocas diárias, podendo permanecer na ferida por até duas semanas.
CONCLUSÕES: O tratamento geral e das lesões na EB é um desafio. A hidrofibra com prata é uma opção de tratamento para as feridas na EB hereditária, sem necessidade de trocas diárias de curativo.
Palavras-chave: Epidermólise bolhosa; Terapêutica; Ferimentos e lesões; Curativos oclusivos; Compostos de prata.
Epidermolysis bullosa (EB) is a group of inherited diseases characterized by skin fragility, with blistering in minimum trauma caused by mutations of several structural proteins in the skin. EB is classified into four types, depending on the location of the mutated protein: simplex, junctional, dystrophic, and Klinder syndrome. There are several subtypes according to the mutation that occurred. The laboratory diagnosis of EB is made by skin biopsy, immunofluorescence antigen mapping, electron microscopy, and mutation analysis1.
More than 1000 different mutations involving 14 structural genes have been described. The mutations cause downregulation of proteins responsible for dermoepidermal adhesion, leading to the formation of blisters on a uniform cleavage plane. The severity of the disease will depend on the protein mutated and the degree of mutation2. EB is a rare disease, with an estimated prevalence of 8.22 per 1 million people and an incidence of 19.6 per 1 million live births. There is no racial or geographical preference3.
The management of EB lesions is a challenge for physicians owing to the complexity and variety of its manifestations. Plastic surgery has an important role in the treatment of complex wounds in EB.
OBJECTIVE
The objectives of this study were to review the general treatment and management of cutaneous lesions in EB, evaluate the indications and effectiveness of hydrofiber with silver, and report the experience of two consecutive patients with EB treated at our institution.
METHODS
A review of the literature was performed on the PubMed/Medline database, including original articles and reviews published between 2009 and 2014. The keywords used were: "epidermolysis bullosa," alone or associated with "congenital," "therapy," "wound care," "inherited'," or "hydrofiber." The most relevant articles were selected, and recommendations of these studies mainly related to management and general injuries were grouped together for summarization.
In addition, we report the case of two consecutive patients with EB treated in the Division of Plastic Surgery, Hospital das Clínicas, Faculty of Medicine of Ribeirão Preto of the University of São Paulo (HCFMRP-USP), conduct adopted, and treatment with the use of hydrofiber with silver (Aquacel® Ag - Convatec®).
RESULTS
General Recommendations
If a neonate is suspected to have an EB, then one should immediately identify the type of lesions by performing a mapping of the affected areas and photographic documentation. Calculation of the affected surface can be estimated using the same methods used for burned patients4. Regular follow-up of the patient should be performed with evaluation of the entire body in search of new lesions, including the scalp, external ear, oral cavity, and genital and anal regions5.
Some general care should be performed with neonates: protection of the bony prominences; avoidance of using an incubator, since heat can lead to the formation of blisters; use of cord ligation rather than umbilical clamps; and avoidance of excessive nasal and oropharyngeal aspiration; however, if it is extremely necessary, soft, low pressure catheters and cushion below the pressure devices may be used; further, adhesives from the electrodes may be removed and fixed with non-adherent bandages.
Clothing should be easy to wear and not contain sewing. Some options in the market, such as Dermasilk® have an antimicrobial activity, which can be used. Diapers should not contain elastics; those with Velcro can prevent adhesive parts from sticking to the skin. Avoid excessive manipulation of the neonate, and when transportation is required, the neonate should be held with one hand of the caregiver behind the neck and another on the buttocks, while avoiding excessive friction. In breastfeeding infants, apply paraffin in the areola and breast to avoid trauma by the search reflex 5.
a) Care of the diaper area: The area can be sanitized with liquid paraffin or oil-based emollient with each change. Primary dressing with soft silicone, such as Mepitel® or hydrogel may also be used; for very exudative lesions, antiseptics or silicone foams, such as Mepilex®5 may be used.
b) Bath: Do not use toxic substances. Bathing can be done with saline solution or water. Acetic acid at a concentration of 0.25 to 1% can be used for better bacterial control6. The bath water should be warm. For heavily infected lesions, 0.1% chlorhexidine can be added. For crustal lesions, emollients or oils may be used. The frequency of the bath should be individualized according to the type and dressing used7,8.
c) Management of blisters: New blisters should be punctured and drained to avoid dissection and extension of the blister; however, the blister skin can be maintained to facilitate re-epithelialization, avoid infection, and reduce pain9,10.
d) Manipulation of crusts: Dried and crusting skin can be treated with emollients, which help reduce the pain and itching10. Keratolytic agents, such as urea and salicylic acid may be used to treat palmoplantar hyperkeratosis; however, special care should be taken in young children11.
e) Care of mucous membranes: The conjunctiva should be regularly lubricated with lanolin and eye drops without preservatives or gel containing hyaluronic acid, polyethylene, or propylene glycol. In addition, nasal lubrication should be performed constantly with products containing vitamin E or simply vaseline12.
f) Nutritional status: The patient with EB in its most severe subtypes was chronically malnourished for several reasons: dysphagia due to ulceration of the oral and esophageal mucosa, microstomia, ankylosis, dental loss, and catabolic consumption due to open lesions. Thus, nutritional support should be initiated early, especially in severe cases of EB. The diet should be hypercaloric (100-150% of the needs) and hyperproteic (115-200% of the needs)13.
Oral or intravenous iron supplementation is indicated in cases of microcytic anemia. Combined use of iron with erythropoietin was proposed in an article14. Some other vitamins, such as vitamins A, C, D, and E, zinc, calcium, carnitine, and selenium can be replenished after evaluation and individualization for each case15.
Enteral nutrition by probe should not be used for long periods, as this can cause erosion in the esophagus and oropharynx. When necessary, the probes should be soft and small. Maximizing nutrition is of vital importance to promote growth and development in children, besides optimizing the healing of lesions16. In some cases, gastrostomy should be indicated to maintain adequate nutritional intake; however, in severe cases as in junctional EB type Herlitz, gastrostomy is not indicated in the palliative context6.
g) Treatment of anemia: In the patient with EB, anemia was multifactorial. There are studies that recommend maintenance of hemoglobin >8 mg/dL6. Hemoglobin levels <10 mg/dL have been described to prolong the healing time of venous ulcers due to reduced oxygenation17. Low levels of hemoglobin in patients with EB are related to delayed healing of the lesions. Oral supplementation of iron can be performed with or without erythropoietin18,19. Blood transfusions may be indicated if hemoglobin levels are <8 mg/dL or for patients not responding to other measures6.
h) Relief of pruritus: Pruritus is a symptom that greatly compromises the patient's quality of life, which is common in EB and is difficult to measure in young children because of their inability to communicate. In older children and adults, it can be treated with antihistamines, such as hydroxyzine, loratadine, and cetirizine. In persistent pruritus, there is a report of treatment with ondansetron and low doses of gabapentin20.
i) Management of pain: Pain is the most common symptom in EB. Pain control should be a part of the daily care of the neonate since in hereditary EB, pain is present from birth. Care should be individualized for each patient, and it is necessary to consider the type of pain.
Measurement of pain intensity is performed by scales for age. Pain can be acute, chronic, or neuropathic. The psychological component may be present in older children. The approach to pain must be both preventive, avoiding traumas, dissemination of blisters, and local infection, and therapeutic. In conducting therapies, the treatment can be pharmacological or non-pharmacological6.
Intense pain is linked to the manipulation of the patient, as in bathing and changing of dressings. Therefore, medications must be used before these procedures, and the type of medication will depend on the type of pain and age of the patient.
Topical anesthetics can be used to minimize pain during dressing and venous punctures; however, attention should be paid to toxic doses. Common painkillers, such as paracetamol are chosen in case of mild to moderate pain. For more intense pain during handling, opioids can be used with or without hydroxyzine, and for control of anxiety and brief sedation, midazolam can also be used.
In children, ketamine is rarely used in the changing of dressings21. For chronic pain, non-hormonal anti-inflammatory drugs may be used. For neuropathic pain, pregabalin, gabapentin, or tricyclic antidepressants may be used together. For acute pain, local anesthetic blocks and botulinum toxin application are described for anal sphincter relaxation in case of erosions and fissures, in addition to paracetamol and opioids. For children under 2 years of age, the use of 24% oral sucrose is described, which promotes short-duration analgesia5,6,22,23.
Categories of wound dressings
The choice of dressing varies according to the location of the lesions and availability of products6,9,10,23,24. Some products decrease the frequency of dressing change and reduce pain and manipulation, which consequently reduce the risk of blister formation and infection. Non-adhesive dressings reduce pain at the time of their removal.
In contrast, some systematic reviews showed only small advantage of advanced dressings (hydrogels, hydrofibers, and foam) when compared with gauze soaked in paraffin for chronic ulcers not related to EB25.
Antibiotics and antiseptics are useful and have a proven role in treating superficial infection in chronic ulcers. Some dressings, such as hydrogel, hydrofiber, and polymer membranes are useful in the debridement of devitalized tissues. However, in case of multiple or deep lesions, surgical debridement with anesthesia may be necessary5.
There are a large number of dressings in the market for the treatment of EB lesions; for each case, the indications should be individualized, since there is no single ideal dressing.
a) Foam: Foam is usually composed of hydrophilic polyurethane; some foams contain silicone to reduce adhesion. It features a semi-permeable membrane, which allows the drainage of exudates. Depending on the amount of exudate, foams can be used up to 7 days, but require continuous change of secondary dressing. Some examples include: Mepilex®, Mepilex Lite®, Mepilex Border®, Mepilex Border Lite®, and Polymem®5,6,24,26.
b) Hydrogels: Hydrogels are made of insoluble polymer, which expands in the presence of water, moisturizing the wound and promoting autolytic debridement. It is indicated for wounds with little or no exudation. Hydrogels improve pain, itching, and discomfort. Some examples include: Duoderm®, Intrasite®, Sheets®, ActFoamCoo®,, and Intrasite Conformable®5,6,24,26,27.
c) Alginate: Alginate is produced by seaweed fibers and transforms into a non-adhesive gel when in contact with exudates. It is indicated for wounds with exudation, with or without association to calcium ions (which promote hemostasis)5,6,28.
d) Modified absorbent dressings: Thin layers of absorbent cotton fiber are placed on layers of polyethylene terephthalate (PET) with the edges sealed with plastic, which prevents adhesion of garments onto the wound; the punctured surface allows the passage of exudates through the dressing. It is relatively inexpensive and non-adherent. Some examples include: Telfa®, Restore®, ETE®, and Mesorb®5,6,24,26.
e) Contact layer: Contact layers are made of inert material and have an atraumatic removal. Some examples include Mepitel®, Silflex®, Mepitac®, and Adaptic touch®5-7.
f) Biosynthetic cellulose: This is a dressing made of cellulose, water, and 0.085% of chlorhexidine gluconate, which both absorbs and moisturizes. Further, biosynthetic cellulose reduces pain and itching. One example is the Suprasorb X®5,6.
g) Lipidocolloid dressings: This dressing is a polyester mesh compound impregnated with hydrocolloid polymers and Vaseline. When in contact with exudates, the hydrocolloid polymers are hydrated and constitute a lipidocolloid interface with petroleum jelly, promoting a non-adherent dressing. It is indicated for wounds with exudates and for protection of vulnerable areas5,6.
h) Hydrofibers: These are composed of carboxy-methylcellulose, which turns into a gel when in contact with exudates, and are more absorbent than alginates. Hydrofibers are indicated for exudative and critically colonized or infected wounds. Aquacel Ag® is a combination of hydrofiber and silver, which acts to control infection. Products containing silver should be used with caution, especially in children, because of its potential toxicity by absorption; further, serum levels should be dosed properly in case of prolonged use. It has limited use in wounds with little or no exudation or with crusts5,6,9,27.
Choice of dressing according to the characteristics of the lesions
a) Dry or slightly exudative wounds: 1) Non-adhesive silicone dressings or lipidocolloid plaques or 2) thin and soft layer of silicone polyurethane and hydrogels can be used. Hydrogels can be changed daily, while the other types can be changed every 3-4 days5-7,9,27.
b) Very exudative wounds: In these cases, hydrophobic or superabsorbable silicone foam dressings are preferred. In addition, soft silicone sponges and polymeric membranes are indicated. Some of these dressings need another secondary dressing, since they do not adhere well5-9.
c) Critically colonized and infected wounds: The diagnosis of infection of the lesions may be performed using a few parameters: delay in healing, increase in the size or quantity of exudates, presence of debris and/or necrotic tissues, strong odor, evaluation of edema, erythema, and increase in temperature. In the presence of the above criteria, some studies suggest swab collection and treatment according to cultures, while others indicate the use of hydrofiber, alginate, and antibiotics for this type of wound5-7,9,24.
d) Pruritus and pain: Biosynthetic cellulose and hydrogel can be used in wounds needing pain and itch control. In addition, topical corticosteroids of medium potency can be used 5,6,9.
e) Hypergranulation: Antibiotic and anti-inflammatory dressings may be beneficial. Further, topical corticosteroids can be used in short periods5-7.
CASE REPORT
In 2014, two patients with EB were admitted at the same time in the Pediatric Intensive Care Unit of the Emergency Unit of HCFMRP-USP. The Division of Plastic Surgery was requested to collaborate in the treatment of these patients with complex wounds resulting from EB.
A 2-month-old male patient (Figures 1, 2 and 3) and 6-month-old female patient had a diagnosis of junctional EB, affecting approximately 38% and 35% of their body surface area, respectively. The lesions affected the head, including the mouth, limbs, trunk, perineum, mucous membranes, and nail beds. Therefore, they had severe cases of the disease with poor prognosis and low survival rates. The lesions were very exudative.

Figure 1. Wounds in the trunk and limbs of the patients with epidermolysis bullosa - right lateral view.

Figure 2. Wounds in the trunk and limbs of the patients with epidermolysis bullosa - left lateral view.

Figure 3. Wounds in the trunk and limbs of the patients with epidermolysis bullosa - front view.
Among the options available in our institution, we chose to use silver hydrogel (Aquacel Ag®), since this dressing is indicated for exudative lesions and silver would be beneficial in controlling the infection, besides not requiring daily changes (Figures 4 and 5). In these cases, dressings were changed according to their saturation.

Figure 4. Dressing of the wounds using hydrofiber with silver - right lateral view.
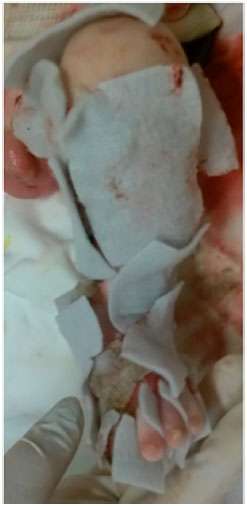
Figure 5. Dressing of the wounds using hydrofiber with silver - detail of the left lower limb.
After applying hydrofiber with silver dressings, we noticed that hypothermia improved, as the dressings in some areas did not require daily changes. There was also reduction in bleeding and apparent better pain control during the dressing changes. We were able to observe healing in some regions, such as the trunk and limbs after the use of hydrofiber.
However, the dressing use was limited in treating the lesions in the perineal region owing to the constant contamination and saturation of the hydrofiber sheaths, needing more constant changes. The two patients died of sepsis between the third and fourth week after dressing application began.
Prospects
Some articles have reported the use of gene therapy, which involved introducing genes into the keratinocyte cells of patients with EB followed by autograft. Transfer has also been studied through retroviral vectors containing genes for expression of type VII collagen in immunodeficient mice. Transplantation of bone marrow and allogeneic blood are also being tested for the treatment of dystrophic EB with some promising results, but requiring more consistent studies to be used on a larger scale29-31.
DISCUSSION
EB is a complex disease, which is often lethal and with devastating effects on the quality of life of patients who survive. The clinical manifestations vary according to each subtype of the disease. The overall treatment of the disease involves nutritional therapy, pain and anemia management, injury prevention, and treatment of injuries in addition to the psychological support of the patient and their families.
Treatment of skin lesions is a challenge for health teams owing to their chronic and relapsing nature. There is scarcity of scientific evidence, with most recommendations based on expert opinions and case reports. There are many options of products in the market for the treatment of injuries resulting from EB. There is no ideal dressing, and the choice must be individualized and based on the characteristics of each lesion, age of the patient, and available resources.
For dry or slightly exudative wounds, most studies suggest hydrogel, lipidocolloid, or non-adherent silicone sheaths. Alginate, hydrofiber, and silicone foams may be used in exudative lesions. In infected lesions, antibiotic ointments, such as silver sulfadiazine or culture-guided parenteral antibiotic therapy may be used.
Hydrofiber with silver is indicated for exudative or infected lesions, and in our experience, it was useful for the control of hypothermia and possibly pain, since the frequency of dressing changes is decreased. One limitation found for its use was in the perineal region because of the frequent presence of urine and feces; with this, layers were rapidly saturated, needing more frequent changes.
CONCLUSION
The overall treatment and lesion management in EB is a challenge. Patient management should be individualized according to the presentation of the disease to minimize physical and psychological sequelae. Hydrofiber with silver is a treatment option for these wounds, as it is useful in controlling hypothermia without the need for daily dressing changes.
COLLABORATIONS
FBC Analysis and/or interpretation of data; conception and design of the study; completion of operations and/or experiments; drafting of the manuscript or critical review of its contents.
PSC Analysis and/or interpretation of data; final approval of the manuscript; drafting of the manuscript or critical review of its contents.
JAFJ Analysis and/or interpretation of data; final approval of the manuscript; conception and design of the study; drafting of the manuscript or critical review of its contents.
REFERENCES
1. Intong LR, Murrell DF. Inherited epidermolysis bullosa: new diagnostic criteria and classification. Clin Dermatol. 2012;30(1):70-7.
2. Fine JD. Inherited epidermolysis bullosa: recent basic and clinical advances. Curr Opin Pediatr. 2010;22(4):453-8.
3. Fine JD, Johnson LB, Suchindran C, Moshell A, Gedde-Dahl T. The epidemiology of inherited EB: findings within American, Canadian, and European study populations. In: Fine JD, Bauer EA, McGuire J, Moshell A, eds. Epidermolysis Bullosa: Clinical, Epidemiologic, and Laboratory Advances, and the Findings of the National Epidermolysis Bullosa Registry. Baltimore: Johns Hopkins University Press; 1999. p. 101-13.
4. Hettiaratchy S, Papini R. Initial management of a major burn: II-assessment and resuscitation. BMJ. 2004;329(7457):101-3.
5. El Hachem M, Zambruno G, Bourdon-Lanoy E, Ciasulli A, Buisson C, Hadj-Rabia S, et al. Multicentre consensus recommendations for skin care in inherited epidermolysis bullosa. Orphanet J Rare Dis. 2014;9:76.
6. Pope E, Lara-Corrales I, Mellerio J, Martinez A, Schultz G, Burrell R, et al. A consensus approach to wound care in epidermolysis bullosa. J Am Acad Dermatol. 2012;67(5):904-17.
7. Mellerio JE, Weiner M, Denyer JE, Pillay EI, Lucky AW, Bruckner A, et al. Medical management of epidermolysis bullosa: Proceedings of the IInd International Symposium on Epidermolysis Bullosa, Santiago, Chile, 2005. Int J Dermatol. 2007;46(8):795-800.
8. Mellerio JE. Infection and colonization in epidermolysis bullosa. Dermatol Clin. 2010;28(2):267-9.
9. Denyer J, Pillay E. Best practice guidelines for skin and wound care in epidermolysis bullosa [citado 2016 Nov 22]. Disponível em: http://www.woundsinternational.com/media/issues/623/files/content_10609.pdf
10. Lara-Corrales I, Arbuckle A, Zarinehbaf S, Pope E. Principles of wound care in patients with epidermolysis bullosa. Pediatr Dermatol. 2010;27(3):229-37.
11. Khan MT. Podiatric management in epidermolysis bullosa. Dermatol Clin. 2010;28(2):325-33.
12. Figueira EC, Murrell DF, Coroneo MT. Ophthalmic involvement in inherited epidermolysis bullosa. Dermatol Clin. 2010;28(1):143-52.
13. Haynes L. Nutrition for children with epidermolysis bullosa. Dermatol Clin. 2010;28(2):289-301.
14. Kuo DJ, Bruckner AL, Jeng MR. Darbepoetin alfa and ferric gluconate ameliorate the anemia associated with recessive dystrophic epidermolysis bullosa. Pediatr Dermatol. 2006;23(6):580-5.
15. Fine JD, Johnson LB, Weiner M, Suchindran C. Gastrointestinal complications of inherited epidermolysis bullosa: cumulative experience of the National Epidermolysis Bullosa Registry. J Pediatr Gastroenterol Nutr. 2008;46(2):147-58.
16. Kim KY, Namgung R, Lee SM, Kim SC, Eun HS, Park MS, et al. Nutritional outcomes in children with epidermolysis bullosa: the experiences of two centers in Korea. Yonsei Med J. 2014;55(1):264-9.
17. Keast DH, Fraser C. Treatment of chronic skin ulcers in individuals with anemia of chronic disease using recombinant human erythropoietin (EPO): a review of four cases. Ostomy Wound Manage. 2004;50(10):64-70.
18. Atherton DJ, Cox I, Hann I. Intravenous iron (III) hydroxide-sucrose complex for anaemia in epidermolysis bullosa. Br J Dermatol. 1999;140(4):773.
19. Fridge JL, Vichinsky EP. Correction of the anemia of epidermolysis bullosa with intravenous iron and erythropoietin. J Pediatr. 1998;132(5):871-3.
20. Allegaert K, Naulaers G. Gabapentin as part of multimodal analgesia in a newborn with epidermolysis bullosa. Paediatr Anaesth. 2010;20(10):972-3.
21. Saroyan JM, Tresgallo ME, Farkouh C, Morel KD, Schechter WS. The use of oral ketamine for analgesia with dressing change in an infant with epidermolysis bullosa: report of a case. Pediatr Dermatol. 2009;26(6):764-6.
22. Anand KJ; International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155(2):173-80.
23. Pope E, Lara-Corrales I, Mellerio JE, Martinez AE, Sibbald C, Sibbald RG. Epidermolysis bullosa and chronic wounds: a model for wound bed preparation of fragile skin. Adv Skin Wound Care. 2013;26(4):177-88.
24. Denyer JE. Wound management for children with epidermolysis bullosa. Dermatol Clin. 2010;28(2):257-64.
25. Chaby G, Senet P, Vaneau M, Martel P, Guillaume JC, Meaume S, et al. Dressings for acute and chronic wounds: a systematic review. Arch Dermatol. 2007;143(10):1297-304.
26. Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37(1):32-9.
27. Schober-Flores C. Epidermolysis bullosa: the challenges of wound care. Dermatol Nurs. 2003;15(2):135-8.
28. Powers JG, Morton LM, Phillips TJ. Dressings for chronic wounds. Dermatol Ther. 2013;26(3):197-206.
29. Siprashvili Z, Nguyen NT, Bezchinsky MY, Marinkovich MP, Lane AT, Khavari PA. Long-term type VII collagen restoration to human epidermolysis bullosa skin tissue. Hum Gene Ther. 2010;21(10):1299-310.
30. Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, Keene DR, Woodley DT, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363(7):629-39.
31. Cutlar L, Greiser U, Wang W. Gene therapy: pursuing restoration of dermal adhesion in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2014;23(1):1-6.
1. Hospital das Clínicas, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brazil
2. Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brazil
Institution: Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brazil.
Corresponding author:
Jayme Adriano Farina Junior
Av. Bandeirantes, 3900 - Monte Alegre
Ribeirão Preto, SP, Brazil Zip Code 14048-900
E-mail: jafarinajr@fmrp.usp.br
Article received: August 25, 2015.
Article accepted: February 10, 2016.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter