

Original Article - Year 2016 - Volume 31 -
Microsurgical head and neck reconstruction: retrospective analysis of 30 free flaps
Reconstrução microcirúrgica em cabeça e pescoço: análise retrospectiva de 30 retalhos livres
ABSTRACT
INTRODUCTION: Reconstructive head and neck surgery is a challenge for the plastic surgeon and requires a large technical arsenal, in which microsurgery plays an essential role. The objective to retrospectively analyze microsurgical head and neck reconstruction performed at the Pontifical Catholic University of Campinas over a period of 2 years.
METHODS: The medical charts of patients who underwent microsurgical reconstruction after head and neck surgery between June 2014 and October 2016 were reviewed to determine which flap was used, the length of the vascular pedicle, recipient vessels, microvascular anastomoses, surgical time, length of hospital stay, complications, and success rate.
RESULTS: Thirty microsurgical reconstructions were performed using 3 types of flaps: anterolateral thigh (n = 15), antebrachial radial (n = 8), and fibula (n = 7). Recipient vessels: facial artery (70%) and facial vein (50%), as 92.4% of microvascular anastomoses were end-to-end. The average operative time was 10.1 hours. The average length of hospital stay was 10.7 days. Two flaps were lost due to arterial thrombosis, resulting in a success rate of 93.3%.
CONCLUSIONS: The microsurgical reconstructions were effective for the repair of complex defects as well as to partially restore the shape and function of the affected tissues. Complications were observed in less than 50% of the cases, although these presented with significant morbidity. The success rate was similar to that reported by major centers of reconstructive microsurgery. The learning curve is long, although it tends to improve with staff training and the acquisition of experience over time.
Keywords: Reconstructive surgical procedures; Microsurgery; Head and neck neoplasms; Surgical flaps.
RESUMO
INTRODUÇÃO: Cirurgia reconstrutiva em cabeça e pescoço representa desafio ao cirurgião plástico e requer amplo arsenal técnico, no qual a microcirurgia figura papel essencial. O objetivo é analisar retrospectivamente as reconstruções microcirúrgicas em cabeça e pescoço realizadas na Pontifícia Universidade Católica de Campinas no período de dois anos.
MÉTODOS: Pacientes submetidos à reconstrução microcirúrgica após cirurgia de cabeça e pescoço, entre junho de 2014 e outubro de 2016, tiveram seus prontuários revisados para avaliação do retalho utilizado, comprimento do pedículo vascular, vasos receptores, anastomoses microvasculares, duração da cirurgia, tempo de internação, complicações e índice de sucesso.
RESULTADOS: Foram realizadas 30 reconstruções microcirúrgicas com três tipos de retalhos: anterolateral da coxa (n = 15), antebraquial radial (n = 8) e fíbula (n = 7). Vasos receptores: artéria facial (70%) e veia facial (50%), sendo que 92,4% das anastomoses microvasculares foram término-terminais. As cirurgias duraram, em média, 10,1 horas. O tempo médio de internação hospitalar foi de 10,7 dias. Houve perda de dois retalhos por trombose arterial, levando à taxa de sucesso de 93,3%.
CONCLUSÕES: As reconstruções microcirúrgicas analisadas foram eficazes na reparação dos defeitos complexos, restaurando parcialmente forma e função dos tecidos acometidos. Foram observadas complicações em menos da metade dos casos, porém com morbidade elevada. O índice de sucesso foi semelhante ao de grandes centros de microcirurgia reconstrutiva. A curva de aprendizado é longa, mas tende a melhorar com o treinamento da equipe e aquisição de experiência ao longo do tempo.
Palavras-chave: Procedimentos cirúrgicos reconstrutivos; Microcirurgia; Neoplasias de cabeça e pescoço; Retalhos cirúrgicos.
Reconstruction performed on the head and neck is a great challenge to plastic surgeons, in view of the functional and 3-dimensional complexity associated with potential skin deficiency, as well as the inadequacy of mucosa, bone, and facial muscles. Fundamental functions such as breathing and swallowing as well as facial expression and speech may be compromised after the removal of a tumor in these areas. Therefore, evaluating possible surgical defects is an essential step to plan reconstruction1.
There are several options for the repair of head and neck defects, including primary closure, skin grafting, and local and pedicled flap2. Until the 1980s, pedicled flaps were considered the preferred option to cover surgical defects. However, they present several limitations, such as limited arc of rotation, necrosis of the distal portion, venous congestion due to pedicle compression, large volume of flap for the area to be covered, and significant injury to the donor area3. In addition, reconstruction of a bone defect with a pedicle flap is practically impossible4.
In the 1990s, advances in microvascular surgery techniques enabled free flap transfers to gain popularity. This was due to the high success rates and the ability to remove large amounts of tissue, intact or not, on a small axis of rotation in the region of microvascular anastomosis5.
Despite the numerous advantages of microsurgical reconstruction, the mastery of this technique demands much sacrifice and a long and difficult learning curve, since the consequences of failure are directly proportional to the complexity of the technique6,7.
OBJECTIVE
To retrospectively analyze microsurgical head and neck reconstructions performed at the Pontifical Catholic University of Campinas (PUC-Campinas), SP, over a period of 2 years.
METHODS
This retrospective study was conducted according to the ethical principles of the Declaration of Helsinki8.
The medical charts of patients who underwent microsurgical reconstruction after head and neck surgery at the Hospital e Maternidade Celso Pierro (HMCP) of PUC-Campinas between June 2014 and October 2016 were reviewed, to determine which flap was used, the length of the vascular pedicle, recipient vessels, microvascular anastomoses, surgical time, duration of hospital stay, complications, and success rates.
Surgical Technique
The author of the study was the surgeon responsible for most of the microsurgical reconstruction procedures. The dissection of the flap, usually performed with 2.5× magnification, was performed along with the removal of the tumor. This was followed by the preparation of recipient vessels, flap transfer and placement, and microvascular anastomoses. In cases of late reconstruction, a cervicotomy was performed along with the dissection and identification of recipient vessels, posterior dissection of the flap, transfer, and microvascular anastomosis.
Recipient vessels were chosen according to the similarity between their diameters in relation to those of the flap. In general, we chose between facial or upper thyroid arteries. However, the vein was usually chosen from among branches of the thyrolinguofacial trunk, external jugular vein, and occasionally the superficial temporal vein. Vascular stumps were prepared for anastomosis using a Zeiss S8 OPMI Vario® surgical microscope. The position of the vessels was established in order to avoid twists, rotations, excessive traction, or compression caused by the flap itself.
Approximately 30 minutes before the sectioning of the pedicle flap, the patient received 2,000 IU of heparin sodium intravenously. At the onset of ischemia, the flap was washed with approximately 100 ml of warm saline solution with 5,000 IU of heparin sodium through cannulation of the flap artery using a 24-G catheter.
Whenever possible, an end-to-end anastomosis was performed, using 4 separate sutures of 10.0 nylon thread (Ethicon® W2850). When the anastomosis was completed, we waited for 5 minutes to review possible leaks, and placed moist gauze under the anastomosis. If a leak was detected, we placed new sutures without clamping.
Then, we performed a patency test using 2 microsurgery forceps. If there was any change, we performed a new anastomosis; this was repeated as many times as the surgeon believed it was necessary. If the problem persisted, we changed the recipient vessel. Whenever possible, the mean arterial pressure was maintained above 70 mmHg without using vasopressors, which are considered deleterious for the microcirculation in the flap.
Although there is no consensus regarding prophylaxis for microvascular thrombosis during anastomosis9, all patients in this study received 20 mg of enoxaparin sodium (Clexane®) subcutaneously for the first 3 days, and 200 mg of oral acetylsalicylic acid (ASA) for 30 days.
The patients were kept in the intensive care unit (ICU) with the doctor on call present, to be able to monitor hemodynamic parameters in the early postoperative period. The flaps were evaluated approximately every 6 hours by means of clinical color parameters, venous return to digital pressure, and bleeding in response to a 25-G needle puncture.
RESULTS
The medical records of 28 patients who underwent microsurgical reconstruction in the Department of Head and Neck Surgery at HMCP of PUC-Campinas between June 2014 and October 2016 were analyzed. Two patients required 2 flaps, one as a consequence of tumor recurrence and the other because of arterial thrombosis of the first flap. This resulted in a total of 30 microsurgical reconstruction cases (Table 1).
Of 28 patients, 18 (67.9%) were males and 10 (32.1%) were females. The average age was 57.1 years, ranging from 18 to 85 years. The main comorbidities were: smoking (55.2%), alcoholism (24.1%), hypertension (20.7%), diabetes mellitus (6.9%), and hypothyroidism (6.9%). Only 3 patients (10%) had previously undergone radiotherapy.
Squamous cell carcinoma (SCC) was the most common diagnosis (58.6%), followed by sequelae of previous surgeries (17.2%), basal cell carcinoma (3.4%), adenocarcinoma (3.4%), adenoid cystic carcinoma (3.4%), melanoma (3.4%), sarcoma (3.4%), ameloblastoma (3,4%), and primitive neuroectodermal carcinoma (3.4%). Most of the reconstructions (80%) were performed immediately after tumor resection, leaving only 20% of the cases for late reconstruction. The surgical defects created by ablative surgeries affected mainly the soft tissues (26.6%), jaw (23.3%), tongue (20%), esophagus (10%), nose (6.6%), maxilla (6.6%), lips (3.3%), and scalp (3.3%).
Of 30 flaps dissected, 15 originated from the anterolateral thigh (ALT), 8 from the antebrachial radial (ABR) region, and 7 from the fibula. The mean length of the vascular pedicles was 15.3 cm from the ALT, 12.5 cm from the ABR, and 2.8 cm from the fibula. In this last flap, the excess proximal bone was discarded, which resulted in a longer vascular pedicle. Therefore, the final mean length of the vascular pedicle of the fibula flap was 8.4 cm.
In all flaps, an arterial anastomosis was performed (n = 30), while in 6 of 8 ABR flaps we performed 2 venous anastomoses, for a total of 36 anastomosed veins. Recipient arteries comprised facial arteries in 80% (n = 21) of the cases, followed by upper thyroid arteries in 23.3% (n = 7), internal thoracic arteries in 3.3% (n = 1) and pharyngeal ascending arteries in 3.3% (n = 1).
Recipient veins comprised facial veins in 50% of the cases (n = 18), followed by external jugular veins in 22.2% (n = 8), internal jugular veins in 16.6% (n = 6), thyrolingual trunk veins in 5.5% (n = 2), superficial temporal veins in 2.7% (n = 1), and internal thoracic veins in 2.7% (n = 1). All arterial anastomoses were end-to-end (EE); however, of 36 venous anastomoses, 13.8% (n = 5) were end-to-side (ES).
The minimum duration of surgery was 5 hours and 45 minutes, while the maximum was 13 hours and 30 minutes, with an average duration of 10 hours and 6 minutes. The patients stayed in the hospital between 4 and 44 days, and the average hospital stay was 10.7 days. Of 28 operated patients, 27 spent an average of 3 days in the ICU. Twelve adverse events were observed: hematoma (n = 3), pneumonia (n = 3), salivary fistula (n = 2), arterial thrombosis (n = 2), partial flap necrosis (n = 1), and dehiscence of the donor area (n = 1). Two patients developed pneumonia and died.
Finally, 28 flaps remained viable, for a success rate of 93.3% (Figure 1).

ALT: Anterolateral thigh; ABR: Antebrachial radical.
Figure 1. Microsurgical flaps performed and success rate.
Figures 2 to 12 illustrate some of the microsurgical reconstructions of this case series.

Figure 2. Donor area of the antebrachial radial flap.

Figure 3. Antebrachial radial flap for nasal reconstruction.
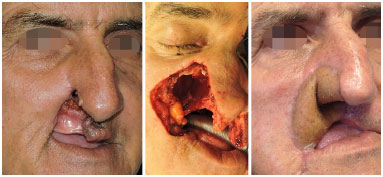
Figure 4. Antebrachial radial flap for lip and nose reconstruction.
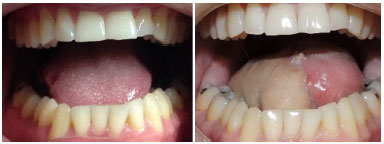
Figure 5. Antebrachial radial flap for tongue reconstruction.
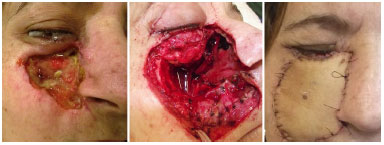
Figure 6. Antebrachial radial flap for face reconstruction.

Figure 7. Donor area of the anterolateral thigh flap.

Figure 8. Anterolateral thigh flap for face reconstruction.
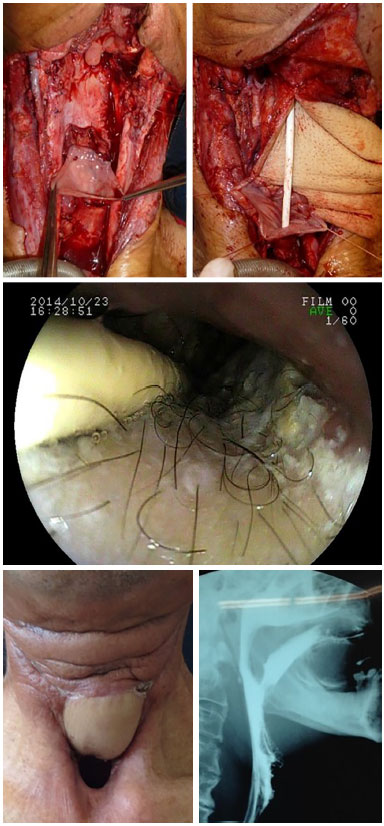
Figure 9. Anterolateral thigh flap for esophagus reconstruction.

Figure 10. Donor area of the fibula flap. Left leg and flap following osteotomy; excess bone discarded, with fixation on a precast titanium mandibular plate.

Figure 11. Fibula flap for left jaw reconstruction following removal of an ameloblastoma.

Figure 12. Fibula flap for mandibular anterior arch defect.
DISCUSSION
Immediate or late sequelae following ablative head and neck surgery are always a challenge to the plastic surgeon, especially with the need to consider functional rehabilitation. In this context, it is essential to add a free tissue transfer procedure to the therapeutic arsenal. Over the past 50 years, several advances in microsurgical techniques and a variety of potential flaps have been described7,10.
The areas that most frequently require microsurgical reconstruction are located in the head and neck, as SCC is the most common malignancy in this area11. In this study, SCC was diagnosed in 58.6% of the cases. Smoking and alcoholism are the most common comorbidities, as also reported in other studies12. Although preoperative radiotherapy is associated with an increased risk of adverse events13, two patients in this study who were previously irradiated did not have any complications.
Studies involving microsurgical reconstruction focus on the greater prevalence of soft tissue and bone defects. Fasciocutaneous and osteocutaneous flaps are most often used to treat these defects5.
In 1974, the first case of free-flap transfer with successful microsurgical vascular anastomosis was reported14. Since then, several flap types have been reported. These include ALT, ABR, and fibula flaps, due to their versatility and wide applicability15,16.
The ALT flap was described in 1984, and is based on a network of lateral femoral circumflex vessels17. Its first use in the head and neck dates back to 199318. This is a versatile fasciocutaneous flap with a long pedicle that does not compromise the main vessel. It has a fatty thigh layer that can serve to treat important volumetric defects16. However, its cutaneous perforators significantly vary from an anatomical point of view. Since most of these are intramuscular, the dissection requires more work19.
The ABR flap was described in 1981 for cervicofacial reconstruction20. Its advantages rely on a thinner cutaneous component, flexibility, and a long and constant vascular pedicle based on the radial vessels. Venous drainage occurs through the superficial system, i.e., the cephalic vein, and the deep system, through the radial constituent veins. The latter is the dominant drainage system21.
The double drainage system explains why some ABR flaps included in the study were performed with two venous anastomoses. The donor area on the forearm should be assessed with the Allen test22. Disadvantages include sacrificing the radial artery, frequent need to graft the skin in order to cover the donor area, and an unsightly scar on the anterior surface of the forearm, besides the risk of complications that might occur in this region23.
The use of the fibula as a possible vascularized bone graft was described in 197524. However, it was only in 1989 that Hidalgo reported its use for mandibular reconstruction25. Since the proximal and distal segments are preserved, its removal does not compromise gait. The fibula is a straight bone that can assume a mandibular shape for dental implants following osteotomy, once the bone has healed in the recipient bed.
This is a chimeric bone flap that might include the gastrocnemius muscle for filling, and skin, to enable monitoring of perfusion in the oral mucosa. The disadvantages of this flap are: a short pedicle, laborious dissection, high morbidity of the donor area, and difficult molding to the skeleton of the lower face in order to maintain good oral occlusion26.
In most cases evaluated in this series, the defect involved soft tissues, thus justifying the use of 23 fasciocutaneous flaps. Whenever thicker skin and larger volume were required for filling, we chose the ALT flap. Conversely, when thinner and more flexible skin was required, with no volume, we chose the ABR flap, as in the case of nose, lip, and tongue reconstruction. However, for 7 cases in which the greatest defect involved the jaw, we chose to perform a microsurgical reconstruction with an osteocutaneous fibula flap, which was then fixed using a titanium plate and screws.
The vascular pedicles described in this study had an average length similar to that reported in the literature21,22,27. However, it was possible to increase the length of the ALT pedicle by dissecting and ligating other branches of the femoral circumflex system. The fibula, which has a short pedicle, was capable of vascular stretching, through distal branch ligation of the soleus muscle vein, and the unnecessary portion of the proximal fibular flap was discarded.
Since Carrel's triangulation technique was first described28, adequate microsurgical technique has been essential for maintenance of visible patent vessels, either through a simple or continuous suture. It is known that poor coaptation of small vessels can lead to swirling blood flow, thus resulting in thrombosis29. End-to-side anastomosis offers a greater technical challenge; however, it is the option of choice in the absence of available recipient veins other than internal jugulars. Therefore, most cases described in this study underwent end-to-end microvascular anastomosis with simple and separate sutures.
The adequate choice of recipient vessels and atraumatic dissection is fundamental for the success of anastomotic patency and good tissue perfusion. The proximity to the location of the defect and the caliber and diameter compatibility between recipient vessels and the vascular pedicle, as well as the integrity of the intima layer, should be taken into account30. Whenever possible, in the cases described herein, these rules were respected.
In the first phase of the learning curve in microsurgical skills acquisition, the focus is on ensuring the vascular viability of the flap, which depends on the surgical technique performed. Long surgical times are common at this early stage of training, and these persist until there is synchrony between all members of the team31. In this case series, we observed that the duration of surgery tended to decrease with time and experience.
The support of the ICU for hemodynamic control and flap monitoring is of great value during the early postoperative period, i.e., when the clinical observation of flap perfusion is essential to diagnose possible changes32. By combining length of stay in the ICU with that in the ward, a prolonged hospital stay is the common denominator for microsurgical reconstructions. However, an average hospital stay of 10.7 days, as observed herein, is shorter than that reported by Al-Dam et al.33.
Prior radiotherapy and prolonged anesthesia, i.e., lasting more than 10 hours, are correlated with an increased risk of complications34; moreover, age over 70 is correlated with increased severity of complications35. In this survey, the 2 patients that died were elderly (74 and 85 years old, respectively). During their prolonged hospitalization, they developed pneumonia, kidney failure, and cardiopulmonary dysfunction. However, their flaps were viable.
Partial necrosis of the ALT flap required a subsequent surgical revision, which required its re-utilization. The donor area of a different ALT developed dehiscence, and the wound was closed without dressings. The 3 cases of hematoma were clinically treated and resolved without surgery.
A low output fistula was observed in an ALT flap that was tubularized for the reconstruction of pharyngoesophageal transit. With clinical support, this improved spontaneously. A skin island was lost in an osteocutaneous fibula flap, and was caused by a venous thrombosis due to perforating veins. This finally evolved into a salivary fistula, which then resulted in a hemorrhagic shock. This required emergency surgery for hemostasis and rotation of the sternocleidomastoid muscle flap towards the posterior floor of the mouth and the de-epidermized fasciocutaneous supraclavicular flap, in order to protect the titanium plate that was partially exposed.
In this case, the fibula flap was viable. Two flaps were lost due to arterial thrombosis: an ALT, replaced by a myocutaneous flap of the pectoralis major muscle with the floor of the mouth; and an ABR for nasal reconstruction, which was debrided, with the wound then treated with dressings and an external prosthesis. Microsurgical reconstructions offer the possibility of solving complex problems, but the defects they cause have high complication rates, as described by Costa et al.36, who reported a rate of 34% (infection, hematoma, fistula, and necrosis) in a series of 63 cases.
Rescue reconstruction and defects that develop following microsurgical reconstruction result in a dramatic scenario that requires a pedicled flap, or even another microsurgical flap, in which the new donor area has been carefully selected to minimize morbidity6,7,37.
In this case series, 93.3% of the microsurgical flaps remained viable, which is in agreement with the percentage reported by other reconstructive centers, with success rates of 85% to 94%7,31,33,38.
Faria, a surgeon who has performed more than 1,210 microsurgical flaps, reported in his thesis that despite the difficult learning curve, the rates of complications decrease over time, by acquiring experience31.
CONCLUSION
The microsurgical reconstructions that were analyzed proved to be effective in repairing complex defects and partially restoring the shape and function of the affected tissues. Complications were observed in less than 50% of the cases; however, the morbidity was very high. The success rate was similar to that reported by large reconstructive microsurgery centers. The learning curve is long, but tends to improve over time with the training of the team and experience.
COLLABORATIONS
ACN Performed the surgeries; designed the study; wrote the manuscript.
JFSC Analyzed the data and critically reviewed the manuscript content.
MBNP Interpreted the data; critically reviewed the manuscript content.
JLBA Performed statistical analyses, interpreted the data; critically reviewed the manuscript content.
TRR Wrote and reviewed the manuscript.
FGC Wrote and reviewed the manuscript.
DARP Reviewed the manuscript.
JCMF Critically reviewed the manuscript content; approved the final version of the manuscript.
REFERENCES
1. Cheng MH, Huang JJ. Oral cavity, tongue, and mandibular reconstructions. In: Neligan PC, ed. Plastic surgery. 3rd ed. London: Elsevier Saunders; 2013. p. 307-35.
2. Hurvitz KA, Kobayashi M, Evans GR. Current options in head and neck reconstruction. Plast Reconstr Surg. 2006;118(5):122e-33e.
3. Hsing CY, Wong YK, Wang CP, Wang CC, Jiang RS, Chen FJ, et al. Comparison between free flap and pectoralis major pedicled flap for reconstruction in oral cavity cancer patients--a quality of life analysis. Oral Oncol. 2011;47(6):522-7.
4. Steel BJ, Cope MR. A brief history of vascularized free flaps in the oral and maxillofacial region. J Oral Maxillofac Surg. 2015;73(4):786.e1-11.
5. Kim K, Ibrahim AM, Koolen PG, Markarian MK, Lee BT, Lin SJ. Highest Impact Articles in Microsurgery: A Citation Analysis. J Reconstr Microsurg. 2015;31(7):527-40.
6. Dedivitis RA, Pfuetzenreiter Júnior EG, Lehn CN, Andrade CRA, Silva RANB. Reconstrução de resgate com retalhos pediculados pós falha reconstrutiva em câncer de cabeça e pescoço. Rev Bras Cir Cabeça Pescoço. 2008;37(3):149-51.
7. Wu CC, Lin PY, Chew KY, Kuo YR. Free tissue transfers in head and neck reconstruction: complications, outcomes and strategies for management of flap failure: analysis of 2019 flaps in single institute. Microsurgery. 2014;34(5):339-44.
8. Sousa MSA, Franco MAG, Massud Filho J. A nova declaração de Helsinque e o uso de placebo em estudos clínicos no Brasil: a polêmica continua. Rev Med (São Paulo). 2012;91(3):178-88.
9. Jokuszies A, Herold C, Niederbichler AD, Vogt PM. Anticoagulative strategies in reconstructive surgery-clinical significance and applicability. Ger Med Sci. 2012;10:Doc01.
10. Gerressen M, Pastaschek CI, Riediger D, Hilgers RD, Hölzle F, Noroozi N, et al. Microsurgical free flap reconstructions of head and neck region in 406 cases: a 13-year experience. J Oral Maxillofac Surg. 2013;71(3):628-35.
11. Dedivitis RA, França CM, Mafra ACB, Guimarães FT, Guimarães AV. Características clínico-epidemiológicas no carcinoma espinocelular de boca e orofaringe. Rev Bras Otorrinolaringol. 2004;70(1):35-40.
12. Alvarenga LM, Ruiz MT, Pavarino-Bertelli EC, Ruback MJ, Maniglia JV, Goloni-Bertollo M. Epidemiologic evaluation of head and neck patients in a university hospital of Northwestern São Paulo State. Braz J Otorhinolaryngol. 2008;74(1):68-73.
13. Herle P, Shukla L, Morrison WA, Shayan R. Preoperative radiation and free flap outcomes for head and neck reconstruction: a systematic review and meta-analysis. ANZ J Surg. 2015;85(3):121-7.
14. Harii K, Omori K, Omori S. Successful clinical transfer of ten free flaps by microvascular anastomoses. Plast Reconstr Surg. 1974;53(3):259-70.
15. Soutar DS, McGregor IA. The radial forearm flap in intraoral reconstruction: the experience of 60 consecutive cases. Plast Reconstr Surg. 1986;78(1):1-8.
16. Wei FC, Jain V, Celik N, Chen HC, Chuang DC, Lin CH. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg. 2002;109(7):2219-26.
17. Song YG, Chen GZ, Song YL. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg. 1984;37(2):149-59.
18. Koshima I, Fukuda H, Yamamoto H, Moriguchi T, Soeda S, Ohta S. Free anterolateral thigh flaps for reconstruction of head and neck defects. Plast Reconstr Surg. 1993;92(3):421-8.
19. Kimata Y, Uchiyama K, Ebihara S, Nakatsuka T, Harii K. Anatomic variations and technical problems of the anterolateral thigh flap: a report of 74 cases. Plast Reconstr Surg. 1998;102(5):1517-23.
20. Yang G, Chen B, Gao Y. Forearm free skin flap transplantation. Nat Med J China. 1981;61:139-44.
21. Timmons MJ. The vascular basis of the radial forearm flap. Plast Reconstr Surg. 1986;77(1):80-92.
22. Evans GR, Schusterman MA, Kroll SS, Miller MJ, Reece GP, Robb GL, et al. The radial forearm free flap for head and neck reconstruction: a review. Am J Surg. 1994;168(5):446-50.
23. Swanson E, Boyd JB, Manktelow RT. The radial forearm flap: reconstructive applications and donor-site defects in 35 consecutive patients. Plast Reconstr Surg. 1990;85(2):258-66.
24. Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55(5):533-44.
25. Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84(1):71-9.
26. Hidalgo DA, Pusic AL. Free-flap mandibular reconstruction: a 10-year follow-up study. Plast Reconstr Surg. 2002;110(2):438-49.
27. Ishida LC. Estudo anatômico do retalho perfurante ântero-lateral da coxa [Tese de doutorado]. São Paulo: Faculdade de Medicina. Universidade de São Paulo; 2006.
28. Carrel A. La technique opératoire des anastomoses vasculaires et la transplantation des viscères. Lyon Med. 1902;98:859-64.
29. Morris DJ, Pribaz JJ. The interrupted-continuous microsurgical suture technique. Microsurgery. 1992;13(2):103-5.
30. Yazar S. Selection of recipient vessels in microsurgical free tissue reconstruction of head and neck defects. Microsurgery. 2007;27(7):588-94.
31. Faria JCM. Microcirurgia reconstrutiva: experiência pessoal de 15 anos - 1210 retalhos livres [Tese de doutorado]. São Paulo: Faculdade de Medicina. Universidade de São Paulo; 2009.
32. Disa JJ, Cordeiro PG, Hidalgo DA. Efficacy of conventional monitoring techniques in free tissue transfer: an 11-year experience in 750 consecutive cases. Plast Reconstr Surg. 1999;104(1):97-101.
33. Al-Dam A, Zrnc TA, Hanken H, Riecke B, Eichhorn W, Nourwali I, et al. Outcome of microvascular free flaps in a high-volume training centre. J Craniomaxillofac Surg. 2014;42(7):1178-83.
34. Singh B, Cordeiro PG, Santamaria E, Shaha AR, Pfister DG, Shah JP. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast Reconstr Surg. 1999;103(2):403-11.
35. Pohlenz P, Blessmann M, Heiland M, Blake F, Schmelzle R, Li L. Postoperative complications in 202 cases of microvascular head and neck reconstruction. J Craniomaxillofac Surg. 2007;35(6-7):311-5.
36. Costa H, Guimarães I, Cunha C, Conde A, Luz M, Pinto A, et al. Reconstrução microcirúrgica da cabeça e pescoço. Acta Med Portuguesa. 1998;11(10):855-70.
37. Hidalgo DA, Disa JJ, Cordeiro PG, Hu QY. A review of 716 consecutive free flaps for oncologic surgical defects: refinement in donor-site selection and technique. Plast Reconstr Surg. 1998;102(3):722-32.
38. Eckardt A, Fokas K. Microsurgical reconstruction in the head and neck region: an 18-year experience with 500 consecutive cases. J Craniomaxillofac Surg. 2003;31(4):197-201.
1. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
2. Pontifícia Universidade Católica de Campinas, SP, Brazil
3. Universidade Federal de São Paulo, SP, Brazil
4. Faculdade São Leopoldo Mandic, Campinas, SP, Brazil
5. Universidade de São Paulo, SP, Brazil
6. Universidade Estadual de Campinas, SP, Brazil
Institution: Serviço de Cirurgia de Cabeça e Pescoço, Hospital Universitário Celso Pierro, Pontifícia Universidade Católica de Campinas, SP, Brazil.
Corresponding author:
André Coelho Nepomuceno
Rua Saint Marie, 383, Quadra S, Lote 2 - Bairro Sousas
Campinas, SP, Brazil Zip Code 13105-832
E-mail: andreconep@yahoo.com.br
Article received: July 18, 2015.
Article accepted: September 29, 2015.
Conflicts of interest: none.



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter