

Original Article - Year 2016 - Volume 31 -
Dermatofibrosarcoma protuberans: a single series of 27 consecutive cases
Dermatofibrosarcoma protuberans: série de 27 casos consecutivos
ABSTRACT
INTRODUCTION: Dermatofibrosarcoma protuberans (DFSP) is a rare low-grade malignant tumor of soft tissues characterized by aggressive local infiltration and propensity for local recurrence. This retrospective study analyzed clinical outcomes, recurrence and survival rates after surgical treatment of DFSP.
METHODS: Patients underwent surgery to complete eradicate tumor, and subsequently a close follow-up by clinical examination and ultrasounds surveillance of primary tumor site and corresponding lymph nodes, to detect local or distant recurrence. Surgery invariably included wide excision of tumor, followed by different reconstructive procedures as skin grafting in 23 cases (85%), local flap in 2 patients (7.4%), free flap in 1 case (4%), while primary closure was performed only in one case (4%).
RESULTS: Second surgery was needed in 9 cases (33%) to achieve minimum free-margins of 2-3cm. Other surgical treatments like Mohs Micrographic Surgery, or adjuvant therapies, like radio- or chemotherapy were not used. Free-recurrence lapse among this series of patients varied from 1 to 10 years, with a medium period of 6 years. Local recurrence occurred in 3 patients (11%), and required a further extended surgical excision. A total of 27 patients did not develop distance metastasis during the follow-up.
CONCLUSION: Extended excision is effective to provide a reliable local control of disease, but only if free margins limiting 2-3 cm is confirmed by pathologist. Clinical and ultrasound surveillance during close follow-up provide early detection of eventual local recurrence and of lymph nodes involvement.
Keywords: Dermatofibrosarcoma; Free tissue flaps; Neoplasm recurrence local; Follow-up studies.
RESUMO
INTRODUÇÃO: A dermatofibrosarcoma protuberans (DFSP) é um tumor maligno de baixo grau de partes moles caracterizado por infiltração local agressiva e propenso a recidiva local. Este estudo retrospectivo analisou resultados clínicos, taxas de recidiva e sobrevida após tratamento cirúrgico de DFSP.
MÉTODOS: Pacientes submetidos a cirurgia para erradicação completa do tumor, e subsequente seguimento por exame clínico e vigilância ultrassonográfica de locais primários do tumor e linfonodos correspondentes para detectar recidiva local ou distante. A cirurgia, invariavelmente, incluiu grande excisão do tumor, seguida por procedimentos de reconstrução diferente como enxerto de pele em 23 casos (85%), retalho local em 2 pacientes (7.4%), retalho livre em 1 caso (4%), enquanto a principal sutura foi realizada apenas em um caso (4%).
RESULTADOS: Foi necessária segunda cirurgia em 9 casos (33%) para atingir margens livres mínimas de 2-3 cm. Outros tratamentos cirúrgicos foram utilizados, como cirurgia micrográfica de Mohs, ou terapias adjuvantes, como radioterapia e quimioterapia. Em nossa série de pacientes o intervalo livre de recidiva variou de 1 a 10 anos, com média de 6 anos. A recidiva local ocorreu em 3 pacientes (11%), e necessitou de outras excisões cirúrgicas extensas. Um total de 27 pacientes não desenvolveu metástase distante durante o seguimento.
CONCLUSÃO: A excisão extensa é efetiva para disponibilizar local confiável para controlar a doença, porém somente se limitadas por margens livres de 2-3 cm e confirmadas por patologista. A vigilância clinica e ultrassonografia durante o seguimento permite identificação precoce de eventuais recidivas locais e envolvimento de linfonodos.
Palavras-chave: Dermatofibrossarcoma; Retalhos de tecido biológico; Recidiva local de neoplasia; Seguimentos.
Earliest description of dermatofibrosarcoma protuberans (DFSP) was given by Taylor in 18901. Subsequently Darrier and Ferrand2 reported a case of progressive and recurrent dermatofibroma. Hoffman3 was the first to describe the tendency of this tumor to develop into protruding nodules, and to define this lesion as dermatofibrosarcoma protuberans.
Although rare, DFSP is the most common stromal tumor of cutaneous origin4. Some epidemological studies have reported a mean annual incidence rate between 0.8 and 4.5 cases per million individuals5. Other studies demonstrated that DFSP has a higher incidence rate among individuals aged between 20 and 50 years6.
DFSP is a low-grade malignant tumor constituting less than 0.1% of all malignancies7. Clinically it often masquerades as a benign, indolent tumor on the trunk and extremities. Early dermatofibrosarcoma can be similar to a keloid, an asymptomatic indurated plaque or nodule, if left untreated it will become firm, raised, and ulcerated8.
Microscopically, it extends far beyond assessed clinical margins, spreading locally in dermis, subcutaneous tissue and muscle. The tumor originates within the dermal layer of the skin: progressively it tends to involve the subcutaneous panniculus and to extend directly through local soft tissue. In presence of long standing tumors, it's been observed invasion of underlying fascia, muscle and bone9. It is believed that the cell of origin in DFSP is a dermal stem cell or an undifferentiated mesenchymal cell with fibroblastic, muscular and neurologic features.
In addition to physical examination, MRI or CT may be useful in evaluating extent of tumor spread10. The first immunohistochemical marker identified for DFSP was the CD34 antigen, which is expressed in up to 90% of cases9. Previous studies have revealed that CD34 is also expressed by other sarcomas and benign fibrohistiocytic lesions, including solitary fibrous tumor, sclerotic fibroma, superficial acral fibromyxoma, dermatofibromas10.
Consequently, CD34 may be considered as a non-specific marker for DFSP9,10. Multiple theories have been put forward for the origin of the DFSP, the main etiological factor in the development of DFSP is the presentation of several prior traumas, including surgical scars, trauma scars, burns, radiodermitis, vaccination sites, sites of central venous lines and insect bites11-14. Some studies reported a history of trauma in 13% cases13. Hereditary influence was not found to be significant15.
A t (17;22)(q22;q13) chromosome translocation was identified by Simon et al.16 as the distinguishing cytogenetic alteration responsible for the development of DFSP. Those chromosomal abnormalities lead to an over expression of the platelet derived growth factor ligand (PDGF).
Biological behavior of DFSP is characterized by aggressive local infiltration, leading to a propensity for recurrence17. According to the literature, the local recurrence rate for patients with DFSP who undergo wide local excision of the trunk ranges from 0 to 21%17, instead is more common in the head and neck region, in which it can occur up to 50-75%18. It is estimated that 80% of local recurrences may occur within the first 3 years after primary surgery19.
Despite their locally aggressive behavior, DFSP rarely metastasize to regional lymph nodes and viscera. Distant metastases are reported with rates from 1% to 4%20, related deaths are mentioned by some authors21,22.
The recommended standard treatment for DFSP is wide local excision of tumor-bearing area, including the subjacent fascia and margin of apparently normal tissue in all planes19. The current recommendations of surgical margin is at least 2 cm, preferably 3 of normal tissue from the gross tumor boundary with a three-dimensional resection that includes skin, subcutaneous tissue, and the underlying investing fascia15,17.
Mohs micrographic surgery (MMS) or staged wide excision "Slow Mohs" (with formal histopathological sectioning and delayed reconstruction for complete circumferential peripheral and deep margin assessment) seems to be the most reliable surgical treatment to avoid local recurrence23.
Radiotherapy administered either before or after surgery may significantly reduce the risk of local recurrence in patients who have or who are likely to have close or positive margins24,25. It has proven benefit as postoperative adjuvant radiation therapy in incompletely excised tumours22.
Its use is controversial, however, as few authors26 have speculated an association between the use of radiation therapy and high grade transformation, therefore a close monitoring is recommended if radiation therapy is undertaken. Chemotherapy has no proven benefit in locally aggressive DFSP and its efficacy in metastatic disease is undefined18.
The over expression of the PDGF, characterizing the DFSP, can be fought by targeted molecular therapy. This therapy aims at stopping tumor growth by blocking PDGF activity16. Imatinib mesylate is a potent and selective tyrosine Kinase inhibitor, with efficacy against the PDGF receptors26, it has also been reported to induce complete or partial remissions in patients with advanced or recurrent DFSP26.
It is most commonly used for cases of DFSP that are unresectable, recurrent or metastatic.22 Although not routinely used, there is growing evidence to suggest that adjuvant targeted molecular therapy may be beneficial25.
OBJECTIVE
This study was to investigated outcomes of surgical treatment of dermatofibrosarcoma protuberans.
METHODS
We focused on 27 consecutive cases of DFSP treated in the Department of Plastic and Reconstructive Surgery in Padova University, from 2004 to 2014. Data were collected from the Cancer Registry of Veneto (Italy) and from medical records.
We include men and women treated at our institution at first instant or coming from other services after primary not radical excision.
Exclusion criteria were uncertain histologic diagnosis, follow up data not available, age of tumor onset was registered but it was not considered a limiting variable.
Characteristics of patients, histologic features, anatomical areas of incidence, size of primary lesion, therapeutic approach were reported.
Using a close follow-up, we studied percentage of local and distant recurrence, survival and tumor-related death.
All patients underwent close both clinical and ultrasound monitoring of primary site and corresponding lymph nodes.
RESULTS
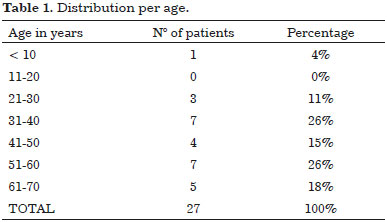
In our series we found that men are more commonly affected than women: 16 men (59%) and 11 women (41%) with a corresponding ratio of 1.4/1. Age ranged from 5 and 70 years, only one pediatric case was excluded, patients mean age was 48 years, while two distinct peaks of incidence are observed in third and fifth decades (Table 1).
Of 27 patients only two came to return consultation after undergoing primary excision in another institute.
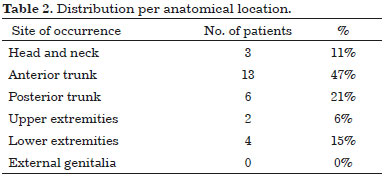
A raised multinodular lesion fixed to overlying skin was primitively the most common clinical figure (96%). Lesion size at time of onset ranges from 1 to 5 cm, greenish-yellow color and firm consistency are salient clinical characteristics in most cases. About half patients, 13 (48%) had lesions confined over the anterior trunk, remaining nodules were distributed on posterior trunk 6 (22%), head and neck 3 (11%), upper extremities 2 (7%) and lower extremities 3 (11%) (Table 2).
No lesions were reported to be sore, nor cause any symptoms except bulking, some patients complained for aesthetical reasons.
At first all patients were led to surgery, radical excision was mandatory but 9 of them subsequently required second surgery to achieve complete tumor extirpation. Two patients required further broader ablation, even after intraoperative sampling was referred free-disease by pathologist.
Because the goal of treatment was radical excision followed by no local recurrence, surgical effort was to ablate tumor with safe margins, therefore width of the resection margin in most of the instances varies from 2-3 cm. Confirmation by pathologist of radical excision was mandatory: histopathology of the resected specimens revealed spindle cells arranged in interwoven pattern and radiating from a focal point (Figure 1 and 2).
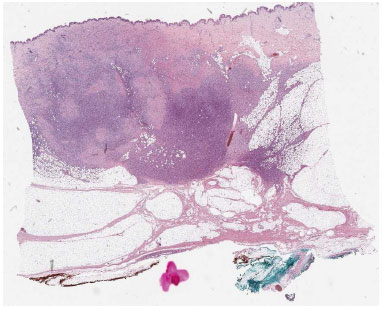
Figure 1. Panoramic view of a dermatofibrosarcoma protuberans infiltranting the hypodermis in a "honeycomb" pattern.
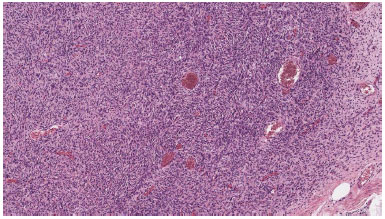
Figure 2. In some areas, the neoplastic cells are arranged in elongated parallel and storiform fascicles.
Tumor cells were arranged in compact bundles, in no tissue sample a perilesional capsule was evidenced (Figure 3). Immunohistochemical figures, when available, showed cells were positive to CD34, negative to S-100, desmin, EMA, ACML, MUC4, P53 (Figure 4).
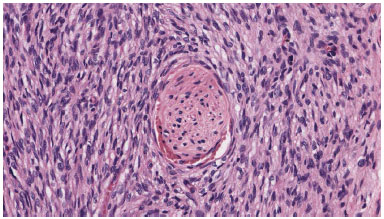
Figure 3. The neoplasm is made of monomorphous fusiform cell that trap a nervous termination.
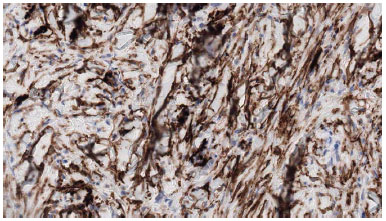
Figure 4. The neoplastic cells express CD34.
The reconstructive ladder depends directly on the site of tumor onset and on width of surgical excision, progression to more complex reconstruction strategies occurs along with defect dimensions getting larger (Table 3).
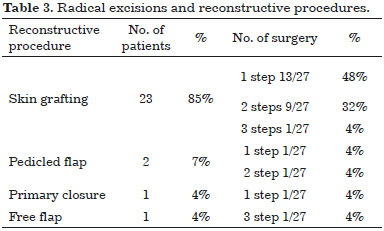
For excision of well-defined masses our choice was skin primary closure, leaving simple linear scar easy to examinate during follow up, that causes patient's minimum inconvenience. When neoplasm's borders were not so well defined or needing second surgery to complete radical resection, the simplest closure was not a viable option.
Oncologic therapy needed extended resection, so the reconstructive planning had to consider proper solutions as skin grafts or local flaps. In particular skin grafting allowed to repair extend resections and same time to detect eventual recurrences. Local flaps were employed both to achieve a better cosmetic results and to repair joints areas like shoulder or ankle.
In details reconstructive procedures included: split thickness skin grafting 23 cases (85%), pedicled flaps in 2 patients (7.4%), free flap in 1 case (free-ALT) (3.7%). In only 1 patient (3.7%) wound could be closed directly by primary approximation of margins.
All patients have been followed up in outpatient visits with both clinical and ultrasound exam of the site of tumor occurrence. Neck, axilla or groin (respectively to primary site) were also scanned to exclude lymph nodes involvement.
No lymph node metastases were detected during follow up. Of the 27 patients studied, 14/27 (52%) remained relapse-free over a period of 12 months to 10 years. Local recurrences occurred in 3 patients (11%), so they underwent to second surgery, but one of them required another operation because of second recurrence.
Out of 27 patients, no patient in the course of follow-up developed distant metastases, 100% of patients remained alive during the follow-up. None of the patients was subjected to adjuvant radio- or chemotherapy.
DISCUSSION
DFSP tumors are characterized by unpredictable and widespread subclinical extension, therefore present high rates of recurrence. The goal remains complete excision of tumor cells with maximum preservation of normal tissue to maintain function and an acceptable aesthetic appearance. The need for clear margins on the face creating potentially large tissue defects poses many reconstructive challenges. The pathogenicity of DFSP is dependent on four risk factors: primary head location, recurrence after previous treatment, depth of tumor invasion, degree of atypical, myxoid or sarcomatous histologic differentiation.
Such number of variables can explain so different outcomes presented in literature. A 5-year cure rates for head and neck sites and for trunk and extremities treated with wide excision reported to be from 25% to 50% and from 80% to 100%, respectively. This could be explained by the surgeons' tendency to take close margins at these sites because reconstructive issues.
In our ten-year study there were 59% men and 41% women corresponding to distribution reported by other authors1,27. Peak age of incidence was observed in third decade of life: only 15% were younger than 30 years-old, remaining 85% were older. Our observation agrees with the literature1,26.
Trunk is the commonest site of DFSP in present series (68%), followed by lower extremities (15%), upper extremities (6%), and head and neck (11%). This observation is in agreement with Taylor and Helwig1, and Burkhard et al.27. DFSP occurred at the rate of 2.6 cases per year, this rate is higher than reported by Roses et al.21.
This increase of incidence may be because patients are currently better educated, healthier conscious than before, and hence they frequently seek medical advice for this tumor more often than in the past. If the tumor is small, the wound can be generally closed primarily without skin grafting.
Skin grafting should be used to close wide defects after radical excision. Recurrence rate is expected to be low if tumor is excised early and with adequate margins. To provide functional results sometimes more complex reconstruction using local or distant flaps is need, especially when joints or pressure areas are involved.
Confidence both with ablation techniques and with reconstructive surgery of high complexity is necessary to achieve satisfaction results, because repairing using flaps after not radical excision would invariably lead to recurrence hard to detect.
Recurrence rate as reported by Pack and Tabah15 and Taylor and Helwig1 was 20.5% and 49%, respectively. Burkhardt et al.27 reported recurrence rate of 33% among DFSP patients who had initial surgery at their clinic. In a series of 27 patients of DFSP by McPeak et al.19 recurrence rate was 11.11%. Roses et al.21 reported a recurrence rate of 32% (41%) when resected margin was < 2 cm and 24% when the resection margin exceeded 2 cm.
Treatment in all the instances consisted in excision with removal of margins of normal skin from macroscopic borders of tumor and in the biopsy to underlying fascia.
The recurrence rate in our series is lower when compared to that reported by Taylor and Helwig1 and Roses et al.21. The reason for lower recurrence rate in our study may depend on wider margins of resection. Close disease-free margins lead to higher rate of recurrence, as indicated by Roses et al.21. In literature it is reported that 3 cm wide margin decreases significantly the rate of recurrence27. Mohs' micrographic surgery, which focuses on resection with precise preservation of normal tissues, also shows low recurrence rate23.
In the present study, none of the patients had lymph node involvement and this finding is concordant with that of Taylor and Helwig who also did not report any lymph node metastasis1. McPeak et al.19 in their study found that in no lymph node metastasis was found when groin and axillary dissection was performed in continuity with excision of primary tumor: clinically enlarged lymph nodes had no evidence of metastasis on microscopy. This reinforces the concept that lymphatic spread does not generally occur in these tumors.
There are few references of the role of radiotherapy in the management of DFSP. At Ontario Cancer Institute, 19 patients of DFSP were managed by surgery and radiation as described by O'Sullivan et al.22: there were two case of local recurrence, those patients underwent salvage surgery.
Although surgery is the gold standard in the treatment, adjuvant radiotherapy in the dose of 50-60 Gy is effective in preventing local regrowth of DFSP following resection with positive margins. Chang et al.28 published about their experience on the role of radiotherapy in treatment of DFSP: they concluded that postoperative radiotherapy can decrease the local recurrence rate in DFSP.
In our study no patients were treated with radiotherapy. This was possible because of radical surgery, while maintaining safe margins, and the close cooperation with the pathologists.
Close clinical and ultrasound follow-up guaranteed absence of local recurrence.
CONCLUSION
Results of our single series confirm that dermatofibrosarcoma protuberans behaves like a locally infiltrating neoplasm. Adequate surgical therapy should be directed towards wide radical excision of primary lesion: resection should be extended to at least 2-3 cm of surrounding margins and the underlying deep fascia must be included in the specimen.
In our study low rates of recurrence without any case of distance metastasis confirmed efficacy of surgical treatment, even without postoperative RT.
After pathologist confirmed surgical margins are free of disease, a close follow-up with half-yearly clinical visits and soft tissue and lymph nodes ultrasound investigation are reliable to warrant effective oncological surveillance.
COLLABORATIONS
CC Writing the manuscript or critical review of its contents.
TB Writing the manuscript or critical review of its contents.
RS Analysis and/or interpretation of data.
FB Final approval of the manuscript.
REFERENCES
1. Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans. A study of 115 cases. Cancer. 1962;15(4):717-25. DOI: http://dx.doi.org/10.1002/1097-0142(196207/08)15:4<717::AID-CNCR2820150405>3.0.CO;2-2
2. Darrier J, Ferrand M. Dermatofibromes progressifs et recidivants ou fibrosarcomes de la peau. Ann Dermatol Syphiligr (Paris). 1924;5:542-62.
3. Hoffman E. Ueber das Knollentribende fibrosarkm der haut (dermatofibrosarcoma protuberans). Dermatol Z. 1925;43:1-28.
4. Lemm D, Mügge LO, Mentzel T, Höffken K. Current treatment options in dermatofibrosarcoma protuberans. J Cancer Res Clin Oncol. 2009;135(5):653-65. PMID: 19205737
5. Chuang TY, Su WP, Muller SA. Incidence of cutaneous T cell lymphoma and other rare skin cancers in a defined population. J Am Acad Dermatol. 1990;23(2 Pt 1):254-6.
6. Sanmartín O, Llombart B, López-Guerrero JA, Serra C, Requena C, Guillén C. Dermatofibrosarcoma protuberans. Actas Dermosifiliogr. 2007;98(2):77-87.
7. Elgart GW, Hanly A, Busso M, Spencer JM. Bednar tumor (pigmented dermatofibrosarcoma protuberans) occurring in a site of prior immunization: immunochemical findings and therapy. J Am Acad Dermatol. 1999;40(2 Pt 2):315-7.
8. Lim EK, Lin VCH, Yu JT, Chang HC. Suprapubic Dermatofibrosarcoma Protuberans: Case Report and Literature Review. J Taiwan Urol Assoc. 2008;19(4):232-4.
9. Aiba S, Tabata N, Ishii H, Ootani H, Tagami H. Dermatofibrosarcoma protuberans is a unique fibrohistiocytic tumour expressing CD34. Br J Dermatol. 1992;127(2):79-84.
10. Kutzner H. Expression of the human progenitor cell antigen CD34 (HPCA-1) distinguishes dermatofibrosarcoma protuberans from fibrous histiocytoma in formalin-fixed, paraffin-embedded tissue. J Am Acad Dermatol. 1993;28(4):613-7. PMID: 7681857
11. Mbonde MP, Amir H, Kitinya JN. Dermatofibrosarcoma protuberans: a clinicopathological study in an African population. East Afr Med J. 1996;73(6):410-3.
12. Bashara ME, Jules KT, Potter GK. Dermatofibrosarcoma protuberans: 4 years after local trauma. J Foot Surg. 1992;31(2):160-5.
13. Tanaka A, Hatoko M, Tada H, Kuwahara M, Iioka H, Niitsuma K. Dermatofibrosarcoma protuberans arising from a burn scar of the axilla. Ann Plast Surg. 2004;52(4):423-5. PMID: 15084891
14. Argiris A, Dardoufas C, Aroni K. Radiotherapy induced soft tissue sarcoma: an unusual case of a dermatofibrosarcoma protuberans. Clin Oncol (R Coll Radiol). 1995;7(1):59-61.
15. Pack GT, Tabah EJ. Dermato-fibrosarcoma protuberans. A report of 39 cases. AMA Arch Surg. 1951;62(3):391-411.
16. Simon MP, Pedeutour F, Sirvent N, Grosgeorge J, Minoletti F, Coindre JM, et al. Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma. Nat Genet. 1997;15(1):95-8. PMID: 8988177
17. Angouridakis N, Kafas P, Jerjes W, Triaridis S, Upile T, Karkavelas G, et al. Dermatofibrosarcoma protuberans with fibrosarcomatous transformation of the head and neck. Head Neck Oncol. 2011;3:5. DOI: http://dx.doi.org/10.1097/00000658-196711000-00011
18. Laskin WB. Dermatofibrosarcoma protuberans. CA Cancer J Clin. 1992;42(2):116-25.
19. McPeak CJ, Cruz T, Nicastri AD. Dermatofibrosarcoma protuberans: an analysis of 86 cases--five with metastasis. Ann Surg. 1967;166(5):803-16. PMID: 4964386
20. Rutgers EJ, Kroon BB, Albus-Lutter CE, Gortzak E. Dermatofibrosarcoma protuberans: treatment and prognosis. Eur J Surg Oncol. 1992;18(3):241-8. PMID: 1607035
21. Roses DF, Valensi Q, LaTrenta G, Harris MN. Surgical treatment of dermatofibrosarcoma protuberans. Surg Gynecol Obstet. 1986;162(5):449-52. PMID: 3704900
22. O'Sullivan B, Catton C, Bell R, Fornasier V, Cummings B, Quirt I, et al. Treatment outcome in dermatofibrosarcoma protuberans referred to a radiation oncology practice. Int J Radiat Oncol Biol Phys. 1995;32(Suppl 1):289.
23. Johnson-Jahangir H, Ratner D. Advances in management of dermatofibrosarcoma protuberans. Dermatol Clin. 2011;29(2):191-200.
24. Williams N, Morris CG, Kirwan JM, Dagan R, Mendenhall WM. Radiotherapy for dermatofibrosarcoma protuberans. Am J Clin Oncol. 2014;37(5):430-2. PMID: 23388563
25. Sun LM, Wang CJ, Huang CC, Leung SW, Chen HC, Fang FM, et al. Dermatofibrosarcoma protuberans: treatment results of 35 cases. Radiother Oncol. 2000;57(2):175-81. PMID: 11054521
26. McGregor JK. Role of surgery in the management of dermatofibrosarcoma protuberans. Ann Surg. 1961;154:255-8. PMID: 13773978
27. Burkhardt BR, Soule EH, Winkelmann RK, Ivins JC. Dermatofibrosarcoma protuberans. Study of fifty-six cases. Am J Surg. 1966;111(5):638-44.
28. Chang CK, Jacobs IA, Salti GI. Outcomes of surgery for dermatofibrosarcoma protuberans. Eur J Surg Oncol. 2004;30(3):341-5.
University of Padua, Padova, Italy
Institution: University of Padua, Italy.
Corresponding author:
Cesare Cappellina
Via 8 Febbraio - 2
Padova, Italy. Zip Code 35122
E-mail: cesarecappellina@gmail.com
Article received: April 15, 2016.
Article accepted: April 27, 2016.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter