

Original Article - Year 2016 - Volume 31 -
Facial filling with polymethylmethacrylate in patients with acquired immunodeficiency syndrome
Preenchimento facial com Polimetilmetacrilato em pacientes que vivem com a síndrome da imunodeficiência adquirida (AIDS)
ABSTRACT
INTRODUCTION: Patients with acquired immunodeficiency syndrome (AIDS) who use highly active antiretroviral therapy (HAART) can develop lipodystrophy syndrome, for which facial filling with polymethylmethacrylate is a treatment option. The objective is to analyze the procedure of facial filling and evaluate patients in relation to their perception, discomfort, revelation of the diagnosis to third parties, expectation concerning facial filling, and satisfaction with the treatment outcome and its impact on their lives.
METHODS: Sixty-three patients who underwent facial filling were evaluated. Procedures performed between January and July 2009 were assessed, the records of the patients were analyzed, and the outpatient lipodystrophy protocol of the STD/AIDS and Viral Hepatitis Municipal Program of São Bernardo do Campo was used.
RESULTS: All the 63 patients who agreed to participate in the research completed the study. Only 6 patients (9.5%) were from other municipalities, while 57 patients (90.5%) were residents of São Bernardo. Of the patients, 68.2% were men and 100% were Caucasian. The mean age of the patients was 49.7 years. Human immunodeficiency virus was diagnosed 11.5 years prior on average, with 10-year average use of HAART and 3.8-year average time of facial lipoatrophy. Most of the patients used stavudine and/or efavirenz. The patients themselves felt more uncomfortable with facial changes. Among the patients, 85.7% did not reveal the diagnosis to third parties.
CONCLUSION: All of the patients were satisfied or very satisfied with the result obtained, which had a favorable impact on their lives. The filling surgical procedure had no adverse effects.
Keywords: Polymethylmethacrylate; Lipodystrophy; HIV; AIDS.
RESUMO
INTRODUÇÃO: Pacientes que vivem com síndrome da imunodeficiência adquirida (AIDS) em uso da Terapia Antirretroviral de Alta Potência (TARV) são suscetíveis a desenvolver síndrome lipodistrófica. O preenchimento facial com polimetilmetacrilato é opção de tratamento. O objetivo é analisar o procedimento de preenchimento facial e avaliar os pacientes em relação à percepção, incômodo, revelação do diagnóstico, expectativa quanto ao preenchimento e a satisfação e impacto em suas vidas.
MÉTODOS: Análise em 63 pacientes submetidos ao preenchimento facial. Foram realizados procedimentos, analisados prontuários dos pacientes e o Protocolo do Ambulatório de Lipodistrofia do Programa Municipal de doenças sexualmente transmissíveis (DST)/AIDS e Hepatites Virais de São Bernardo do Campo, atendidos no período de janeiro a julho de 2009.
RESULTADOS: Todos os 63 pacientes iniciais que concordaram em participar da pesquisa permaneceram até o término deste trabalho. Apenas seis pacientes (9,5%) eram de origem de outros municípios, enquanto 57 pacientes (90,5%) eram moradores de São Bernardo. 68,2% eram homens e 100% brancos. A média das idades foi 49,7 anos. Em média, o Vírus da Imunodeficiência Humana (HIV) foi diagnosticado há 11,5 anos, com tempo médio de uso de TARV por 10 anos e tempo médio de lipoatrofia facial de 3,8 anos. A maioria fez uso de Estavudina e/ou Efavirenz. Quem ficava mais desconfortável com as alterações na face eram os próprios pacientes. 85,7% não revelaram o diagnóstico para terceiros. 100% dos pacientes ficaram satisfeitos ou muito satisfeitos com o resultado obtido.
CONCLUSÃO: 100% dos pacientes ficaram satisfeitos ou muito satisfeitos com o resultado obtido. Em 100% dos casos houve um impacto favorável na vida. Não houve efeitos adversos ao procedimento cirúrgico de preenchimento.
Palavras-chave: Polimetilmetacrilato; Lipodistrofia; HIV; AIDS.
The first cases of acquired immunodeficiency syndrome (AIDS) in Brazil were diagnosed in the 1980s. In this period, AIDS was known as the "5-H disease," alluding to the initials in English, homosexuals, hemophiliacs, Haitians, heroin users (injectable heroin users), and hookers. The possible forms of transmission of the disease were then announced as follows: sexual contact, use of injectable drugs, or exposure to blood and derivatives1.
The first diagnostic tests for human immunodeficiency virus (HIV) infection, based on the detection of antibodies against the virus, were developed in 1985. At the end of this decade, the success of zidovudine (AZT), the first drug to show efficacy, albeit limited, against the progression of immunodeficiency, was divulged. Its use in monotherapy demonstrated efficacy in reducing mortality and the incidence of opportunistic diseases. It was used in monotherapy until 1993, when studies involving new drugs were published2.
In 1996, highly active antiretroviral therapy (HAART), in Portuguese "Terapia Antiretroviral de Alta Potência" (TARP), was introduced, when the efficacy of the association of different classes of drugs was proven, in a sustainable and lasting manner2.
With the implementation of a federal law (No. 9313) in November 1996, the Health Ministry of Brazil began to ensure comprehensive care for people with HIV infection/AIDS, including the free distribution of antiretroviral drugs via the Single Health System3. In 1998, another federal law (No. 9656) included AIDS in the pathologies of mandatory care by private insurance health care, further expanding the possibilities of care to patients with AIDS4.
In the 21st century, the Brazilian program to control HIV infection/AIDS is internationally considered as an example, with its most striking characteristics being the integration between prevention and care, the incorporation of the perspective of civil rights to prevention and universality. It is worth highlighting that of the 18 antiretroviral (ARV) medicines distributed by the SUS, eight are produced nationally5.
The HAART, together with the national public policies that guarantee the free, universal distribution of therapeutic regimens, transformed AIDS into a chronic disease and brought about changes in the prognosis and prolongation of the life expectancy of seropositive people.
As a result, a new aspect began to call the attention of physicians and patients. Patients began to present changes in body fat distribution, with reduction of fat in some peripheral areas of the body such as the upper limbs, lower limbs, and buttocks. These changes were called lipoatrophy (LA). In the facial region, fat loss occurs mainly in the malar (Bichat's fat pad), and pre-auricular and pre-temporal regions, and is named facial lipoatrophy (FL)6.
On the other hand, in the central regions of the body, such as the abdomen and breasts, and the dorsocervical region, the accumulation of fat is called lipohypertrophy (LH). The combination of these lipoatrophy and lipohypertrophy changes was called lipodystrophy, which causes significant morphological changes7.
The first reports of changes in body fat distribution in people with AIDS that are associated with HAART were published in 19988. The etiopathogenesis of this heterogeneous syndrome remains unknown, and the pathogenesis is probably multifactorial9.
Lipodystrophy syndrome (LDS) is considered to have a significant social impact because it reduces the quality of life in those who develop it. It was officially described by the Food and Drug Administration (FDA-USA) in 199710 and received different names over the years. Among these names were "crixbelly," "pseudo Cushing syndrome," "body fat redistribution syndrome," "metabolic syndrome associated with HAART, and "dyslipidemic HIV/HAART-associated lipodystrophy"11. Metabolic changes such as elevated cholesterol, triglyceride, and glucose levels also characterize this syndrome6.
Initially, LDS was associated with the use of HAART, more specifically to protease inhibitors (PI), and subsequently to the use of nucleoside analogue reverse transcriptase inhibitors (NRTI). The latter category is more associated with facial lipoatrophy7.
Subsequently, lipodystrophy was also observed in some patients who had never used HAART, suggesting the involvement of other mechanisms such as inflammatory, genetic, and environmental factors. The adipose tissue plays an important role in the clinical and metabolic aspects of the syndrome, as the mechanisms of adipocyte differentiation are the main targets of action of antiretroviral drugs12.
Lipodystrophy treatment includes the change of antiretroviral therapy, when possible, and/or surgical procedures to minimize the volume changes in the affected areas13.
The risk factors of FL are as follows: exposure to stavudine (D4T) and PI, advanced age, low plasma levels of CD4+ T lymphocytes (CD4+), high plasma viral load (VL), duration of HAART use, and being Caucasian14.
No consensus has been reached in the literature on the prevalence or the incidence of lipodystrophy in patients with AIDS. Different studies suggest that the prevalence and incidence may vary from 6% to 84% and from 7.3 to 11.7 per 100 patients/year, respectively. The prevalence rates tend to increase in accordance with the increase in HAART use15.
As a result of the changes caused by this syndrome, medical teams that provide care to persons with AIDS must ideally include infectologists, dermatologists, and/or plastic surgeons, nurses, psychologists, social assistants, pharmacists, nutritionists, physiotherapists, speech therapists, cardiologists, and endocrinologists, as the presence of a multidisciplinary team significantly improves the management of the disease16.
In Brazil, from 1980 to June 2011 (base year 2010), 608,230 AIDS cases were reported. In 2010, 34,218 new cases were reported, with a national incidence rate of 17.9 per 100,000 inhabitants17. On this occasion, it was estimated that about 180,000 sought AIDS medications in accredited hospitals17. Regarding facial lipoatrophy, no official estimates of the number of patients with this diagnosis have been reported.
All available treatments have contraindications and may cause adverse effects. However, the surgical procedures for cutaneous filling in atrophy areas may benefit the patients by reducing the stigma and contribute to the improvement of their psychological conditions, improve their quality of life, and foster adherence to treatment with HAART18.
OBJECTIVE
The objective of this study was to report and analyze the procedure for facial filling with polymethylmethacrylate (PMMA) in AIDS patients and evaluate the patients before they undergo facial filling in relation to their perception and discomfort caused by facial lipoatrophy, revelation of the diagnosis to third parties, expectation concerning facial filling, and satisfaction of the outcome and its impact on their lives.
METHODS
This was a cross-sectional study of a convenience sample that was conducted in an outpatient clinic that specialized in AIDS patients who participated in the STD/AIDS and Viral Hepatitis Municipal Program of the Municipality of São Bernardo do Campo, São Paulo, Brazil, between January and July 2009.
All the patients who attended the outpatient lipodystrophy clinic within the aforementioned period participated in the study. None of the patients was lost to follow-up.
The sample was composed of 63 AIDS patients with facial lipoatrophy who were clinically assessed by a infectologist in order to rule out other pathologies that would contraindicate the procedure, by a speech therapist for assessment of the oral sensorimotor system and the application of the questionnaire, and by other professionals of the multidisciplinary team, when necessary, for completion of the protocols from the lipodystrophy clinic of the STD/AIDS and Viral hepatitis Program of São Bernardo do Campo, according to the determinations of Decree no. 118 of February 23, 2005, of the Ministry of Health19, later replaced by joint administrative ordinance no. 1. This joint ordinance SAS/SVS No. 01 of January 20, 2009, normalized the completion of facial filling with PMMA in an outpatient level12.
After signing the Free and Informed Consent form, the patients were referred for completion of facial filling with PMMA for correction of facial alterations caused by facial lipoatrophy.
PMMA was applied by a plastic surgeon who received training specifically for this procedure by the Ministry of Health. The patient was placed in the supine position, between 30° and 45°. Topical skin anesthesia with lidocaine hydrochloride 4% (Figure 1A) was administered, together with local cooling with a thermogel bag to 0°C for 20 minutes (Figure 1B). Prior to the applications, markings on the areas to be filled were performed (Figure 1C). After antisepsis with 10% polyvinylpyrrolidone iodine (PVPI), anesthetic blockade of the infraorbital nerves (Figure 2A) and local infiltration with anesthetic buttons was performed in temporal regions (regional blockade; Figure 2B) and medial regions of the branches of the mandible (Figure 2C), with 2% lidocaine hydrochloride associated with epinephrine.
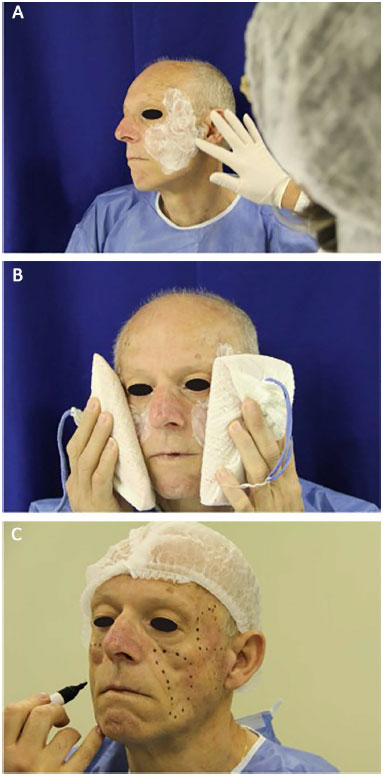
Figure 1. Preparation for application of polymethylmethacrylate (PMMA). A: Application of an anesthetic ointment. B: Pre-anesthesia application of an ice pouch for 20 minutes. C: Planning and marking of areas to be filled.

Figure 2. Anesthesia. A: Infraorbital anesthetic block. B: Regional temporal anesthetic block. C: Anesthetic complementation in the mandibular branch.
The material used for the facial filling was 30% PMMA in the malar regions (Figure 3B) and 10% PMMA in the temporal regions (Figure 3A), in the subdermal layer through retroinjection, as this is the filler determined by the Health Ministry of Brazil. The skin was punctured in regions close to the mandible and temporal region by using a 40- × 12-mm needle to create a micropuncture that allowed the introduction of 50- × 10-mm microcannula in place that could reach the area to be filled.
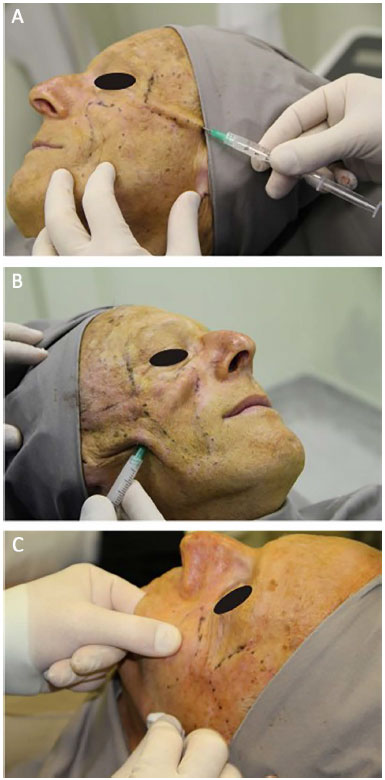
Figure 3. Procedure. A: Facial filling of the temporal region. B: Facial filling of the malar region. C: Massage after filling.
At the end of the procedure, the filled facial region was massaged for better distribution of the PMMA (Figure 3C).
The procedure was concluded with simple dressings in the microcannula puncture regions. At the end of the filling, cold compresses were placed for 20 minutes (Figure 4).
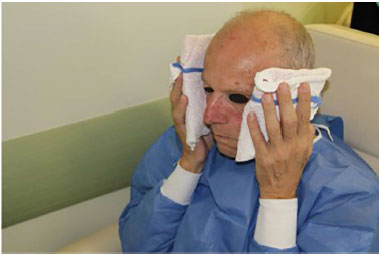
Figure 4. Post procedure: Reapplication of an ice pouch.
The patients received prescription drugs for pain relief, if necessary (paracetamol, every 6 hours).
Many filling steps were performed as necessary for the correction of the deformity caused by facial lipoatrophy. One month after the total correction of facial lipoatrophy, the patients answered the questionnaire again (specific post-fill issues) before discharge from the outpatient clinic.
The inclusion criteria were as follows: having a diagnosis of AIDS and facial lipoatrophy; using antiretroviral therapy for a period of not less than 12 months; without clinical manifestations suggestive of immunodeficiency in the last 6 months; present with a CD4+ T-lymphocyte count (CD4+) of >200 cells/mm3 and VL of <10,000 copies/ml (preferably) that were stable over the past 6 months; clinical and laboratory parameters in accordance with the necessary and sufficient safety criteria for any surgical procedure; age of >18 years; and having signed the Free and Informed Consent and the Consent Term of Use of Photographic Image forms.
The exclusion criteria were as follows: any clinical condition or decompensated comorbidity in the last 6 months that would increase the procedure-related risk; concomitant treatment with anticoagulants, immunomodulators, immunosuppressive drugs, or chemotherapy; and pregnancy or breastfeeding in progress.
Research instruments included the sociodemographic aspects, namely age, sex, ethnicity, and medical-clinical aspects, namely time of HIV diagnosis, use of HAART, and facial lipoatrophy; use of D4T and efavirenz (EVF), as these are the drugs commonly associated with the development of the facial changes; CD4+ T lymphocyte count and current plasma VL, as predictive indicators of health status; presence of dyslipidemia, which would suggest the presence of lipodystrophy syndrome; and use of psychotropic drugs, suggesting the presence of some depressive symptoms. The questionnaire was also administered in the preoperative and postoperative facial filling period, encompassing the following:
Questions regarding pre facial filling:
1. Who perceived the facial lipoatrophy: the patient himself or others?
2. Who is troubled with facial lipoatrophy: the patient himself or others?
3. Revealed the diagnosis to third parties: yes or no?
4. What is the expectation from the facial filling in terms of outcome: very low, low, fair, high, and very high?
Questions regarding post facial filling:
1. What is the satisfaction after the facial filling: dissatisfied, satisfied, or very satisfied?
2. What is the impact on life: favorable or unfavorable?
Finally, the occurrence of complications or adverse events related to the filling procedure or the product used as filler was assessed.
The data were statistically analyzed by using the programs EPI INFO - A Word Processing, Database and Statistics Program for Public Health20, to prepare the database and the Statistical Package for the Social Sciences- SPSS 17.0. The descriptive analysis was performed by means of central tendency and dispersion measures, tables of percentages, and graphics. The medical charts of the patients and the Protocol of the Clinic of Lipodystrophy of the STD/AIDS and Viral Hepatitis Municipal Program of São Bernardo do Campo were used as a source of data collection.
All findings with a p value of <0.05 were considered statistically significant. The adjusted and non-adjusted odds ratio were calculated with their respective 95% confidence intervals as a measure of risk. We consider an odds ratio of 1 as a basic category for each independent variable, the lower risk for the outcome of interest.
RESULTS
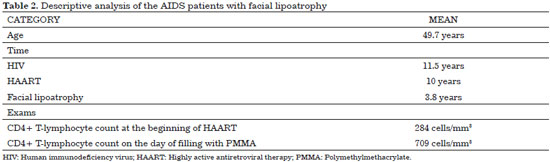
None of the patients were lost to follow-up. All 63 patients who agreed to participate in the study completed the study. Only six patients (9.5%) were from other municipalities, while 57 patients (90.5%) were residents of São Bernardo do Campo. Men (68.2%) and Caucasians (100%) were predominant. The mean age was 49.7 years. The results presented in Tables 1 and 2 provide a description of the sample assessed (Figure 5).
DISCUSSION
The 63 patients evaluated were predominantly male (68.2%) and Caucasian (100%) (Table 1), with a mean age of 49.7 years (range, 34-74 years; Table 2). Although only few studies are related exclusively to facial lipoatrophy, a study conducted in Spain that involved 62 patients with this type of alterations presented a similar distribution, with 71% of the patients being male and with a mean age of 40 years21.
Regarding sex, one study suggests that male patients present a greater tendency to develop lipoatrophy than the opposite sex22. Another study suggests that men seem to be more prone to fat loss in peripheral regions, while women accumulate fat in central regions23, while facial lipoatrophy is an alteration observed in peripheral areas.
No consensus has been reached as to whether ethnicity influences the prevalence or characteristics of lipodystrophy. Fat redistribution appeared to be less prominent in African Americans and in Hispanics than in Caucasians. Other studies performed in Singapore, Japan, and Nigeria showed similar prevalence rates in these three ethnic groups24. During the data collection phase of this study, 100% of the patients with facial lipoatrophy who were referred to the lipodystrophy outpatient clinic were Caucasian (Table 1).
CD4++ T lymphocyte (CD4+) count is a predictor of opportunistic diseases, thus being of great value for patients and infectologists. Very low initial CD4+ count is known to be associated with higher risk of onset of lipodystrophy12.
The postponement of the use of HAART in asymptomatic patients with CD4+ counts close to 350 cells/mm3 has been the strategy used in clinical practice to decrease the time of exposure to these drugs and their adverse effects, as a very low baseline CD4+ count is associated with increased risk of the onset of lipodystrophy25.
The mean CD4+ T lymphocyte count of the patients in this sample was of 709 cells/mm3 at the moment of filling and 284 cells/mm3 at the beginning of the treatment (Table 2). VL was undetectable in 88.9% of the patients (Table 1).
On average, the patients were diagnosed as having HIV seropositivity 11.5 years prior, were receiving HAART for 10 years, and reported the first signs of facial lipoatrophy 3.8 years prior (Table 2).
Studies suggest that Caucasian patients aged >40 years, with HIV infection for >10 years, with initial CD4+ counts of <100 cells/mm3, and who used D4T for any period have higher risks of developing some type of lipodystrophy3.
Patients with facial lipoatrophy and long-standing diagnosis of HIV infection are more frequently men, probably because at the time the patients of this series were diagnosed as having HIV seropositivity, the predominant contamination of men was evident.
In 1985, in relation to the diagnosis of AIDS in accordance with the AIDS and STDS Epidemiological Bulletin 2011, for every 26 men with the infection, one woman also had the infection. In 1990, for every 3.7 men with AIDS, one woman also had AIDS. In 2010, for every 1.4 men with AIDS, one woman also had AIDS.
The frequency of facial lipoatrophy in ambulatory patients with lipodystrophy as defined in the STD/AIDS and Viral Hepatitis Program of Sao Bernardo in this period was 8.43%.
No consensus has been reached on the incidence of lipodystrophy in AIDS patients. This value can vary from 6% to 69% in patients receiving HAART for at least 1 year26.
Regarding the use of HAART, all of the patients used HAART for >1 year, with a mean of 10 years. D4T was used by 88.9% of the patients; and EVF, by 77.8% of the patients. These two ARV medicines were used by 68.2% of the patients but never used by 1.5% of the evaluated patients (Table 1).
Initially, it was common to associate lipodystrophy syndrome in people with HIV infection/AIDS by using HAART. The use of D4T, a nucleoside analog reverse-transcriptase inhibitor (NRTI) was initially the most related with facial lipoatrophy7. Subsequently, the occurrence of lipodystrophy in patients who never used antiretroviral drugs was observed, suggesting the involvement of other mechanisms, including inflammatory, genetic, and environmental factors.
Several studies associated the use of D4T with the occurrence of lipodystrophy and, more specifically, to facial lipoatrophy27. Recent studies show that EVF is also associated with the appearance and progression of lipoatrophy12.
The high rate of patients who used D4T (88.9%) is probably associated with the low availability of antiretroviral drugs in the past. The use of EVF (77.8%) arises from its being one of the first choice drugs for untreated AIDS patients.
The facial changes caused by lipoatrophy entail greater psychological impact and often cause a cadaveric aspect to the face, revealing the diagnosis of seropositivity, which most of the time is meant to remain confidential15. It is therefore a matter of great concern to AIDS patients, especially those in this study, as in this sample, the time of diagnosis of HIV and the use of HAART were longer than 10 years (Table 2).
Of the patients evaluated, 50.8% used some type of psychotropic drugs, initiated only after the diagnosis of HIV (Table 1). A close correlation was observed between body morphological changes and depression in AIDS patients. Facial lipoatrophy was regarded as the most critical triggering factor of the more severe cases of depression28.
Dyslipidemias, as a clinical factor associated with HIV, are increasingly present in people with AIDS. Reports on changes in blood pressure, lipid levels, blood sugar levels, and increased cardiovascular risk are common15.
HIV infection itself, regardless of the use of HAART, is known to be associated with dyslipidemias. Soon after the onset of HIV infection, HDL cholesterol and triglyceride levels decrease but increase with decreasing levels of total cholesterol and LDL cholesterol. With the introduction of HAART, total cholesterol, LDL cholesterol, and triglyceride levels gradually increase with HDL cholesterol levels regaining its level before the initiation of antiretroviral medication23. Of the patients evaluated, 74.6% presented with dyslipidemia associated with facial lipoatrophy (Table 1).
Regardless of ethnicity, sex, or age range, facial lipoatrophy is an increasingly frequent theme in infectiological services and is considered a revelation of the diagnosis of AIDS to third others, which patients are highly concerned to avoid. In this sample, 85.7% of the assessed patients did not reveal the HIV diagnosis to third parties (Table 1). This is directly associated to the stigma and prejudice related to the disease, which has always accompanied the history of AIDS, throughout the evolution of the epidemic29.
Another negative implication of facial lipoatrophy is its impact on treatment adherence, functioning as a disincentive. The facial, body, and life style changes cause significant discomfort, leading many patients to abandon treatment15. However, all the patients who underwent facial filling continued using HAART, without interrupting or abandoning the treatment until the completion of this study.
As facial lipoatrophy does not have a cure, the surgical procedures for cutaneous filling in areas with atrophy may benefit the patient to mitigate the stigma. The treatment of facial lipoatrophy in patients with HIV/AIDS may contribute to the improvement of their psychological condition, improve the quality of life and foster adherence to treatment with HAART30.
Currently, the methods of restoring facial volume include the use of filler agents18 such as PMMA, which was approved by the Ministry of Health in Brazil. Medical literatures agree that an ideal strategy is lacking for the treatment of these patients, as they all show some contraindication and may cause adverse effects even when taking into account the inclusion and exclusion criteria31.
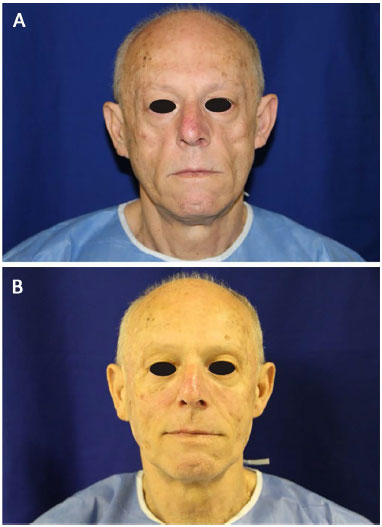
Facial filling with PMMA in AIDS patients with facial lipoatrophy provides, in general, satisfactory esthetic results. One of the greatest advantages is the regaining of patients' self-esteem after once again recognizing themselves in the mirror12.
Thus, the discomfort and the implications of facial lipoatrophy go beyond any question or esthetics of revelation of the diagnosis, which is why the percentage of patients who say they are uncomfortable with the facial change is as high, and so is the expectation with regard to filling. In this sample, 79.4% of the patients who submitted to facial filling had high or very high expectations with regard to the treatment results (Table 1).
The Brazilian Ministry of Health, after judicious studies, has long approved the use of PMMA for completion of facial filling by medical specialists in the area of plastic surgery or dermatology in patients with AIDS and those receiving HAART for a period of not <12 months.
Since the discovery of HIV, management of the disease has evolved considerably and the initiatives to prevent HIV infection and improve treatment and quality of life of AIDS patients have increased. Facial lipoatrophy, by changing the aspect of the face, has a negative impact on the life of a patient with AIDS. In these cases, facial filling is more than an esthetic intervention, encouraging treatment adherence. This is because patients, when they are more satisfied with their physical appearance, comply with the medical prescriptions with fewer questions and more easily follow the proposed treatment.
Metacril®, a PMMA manufactured by the Nutricell Laboratory, is the product supplied by the State Coordination (STD/AIDS Reference and Training Center of São Paulo) for facial filling. It is composed of microspheres with diameters of >30 microns. These microspheres are suspended in a colloid medium of carboxymethylcellulose, which acts as a carrier. The diameter of the microspheres, which is greater than the size of the macrophage, prevents them from being phagocytized.
After injection, the colloid medium is absorbed by the body. Proliferation of fibroblasts then occurs, which leads to the microencapsulation of microspheres, with reduced foreign body reaction, which prevents their migration. This can be observed in the histological examination. These characteristics make PMMA a biocompatible, permanent, and long-lasting filler with prolonged results12.
PPMA was chosen as filler for AIDS patients with facial lipoatrophy after 10 years of follow-up because it is a safe product, involving a relatively simple method of application and best cost-to-benefit ratio when compared with other cutaneous filler materials12.
Few studies have reported adverse effects related to the use of PMMA16.
These adverse effects that are related to the various types of fillers, when they occur, refer to edema, ecchymosis, redness, pain, nodules, or tangible micronodules in the subcutaneous tissue, but not visible and, in general, disappear spontaneously in a short period of time32.
Nodules, granulomas, and hyperemia were described in the malar and submalar regions33.
In the present study, the adverse effects that occurred were transient. Of the patients, 4.8% presented accentuated local edema shortly after filling and 19% had ecchymosis in the region of the infraorbital nerve block, which disappeared within 7 to 14 days (Table 1). One of the factors that contributed to the low incidence rate of side effects was the use of a microcannula. Microcannulas are longer than needles, which allows the filling to be performed with a smaller number of punctures and with lower injection pressure.
Owing to the atraumatic tip of microcannulas, lesions in blood vessels or changes in the filling layer less likely occur, which makes the procedure safer, with lower risks of hematomas, edema, and pain34. Using microcannulas also lowers the risk of injection of the product in the blood vessels, which could lead to embolism with consequent tissue necrosis. Another factor that contributed to the low occurrence of adverse effects in this study was the previous training that the Ministry of Health offered to plastic surgeons and dermatologists.
Facial lipoatrophy is considered the most significant change from the psychological point of view. Therefore, in this study, when questioned, 85.7% of the patients reported not divulging its diagnosis to others (Table 1).
Thus, to evaluate the degree of satisfaction of patients in relation to the treatment performed at our clinic, they were interviewed about the inconvenience that lipoatrophy caused them before the intervention.
Of the patients who underwent facial filling, 19% were satisfied and 81% very satisfied with the results obtained, with 100% favorable impact in their lives, in agreement with published reports16 of improvement of the appearance and support of the recovery of patients' identities and dignities, minimizing discrimination and prejudice.
The answers provided by the patients further showed that 74.6% of the patients themselves noticed the change caused by facial lipoatrophy and that 76.2% of the time, the patients felt discomfort with this situation (Table 1).
Even in cases in which the perception of facial alteration was observed by the physician or partner, the patient realized the positive facial change after the completion of the filling and returned after the first application to continue treatment. This suggests that even when the discomfort was not verbalized, they could see the improvement obtained after the intervention.
These indexes of approval are considerable, as reports in the literature indicated that 28.3% of patients receiving HAART were afraid of developing facial lipoatrophy35.
In this study, 79.4% of the patients reported that their expectation regarding the result of the facial filling was high or very high (Table 1). After the procedure, 81% of the patients were very satisfied and 19% were satisfied (Table 1). These data corroborate the statements that after treatment completion, patients once again recognize themselves in the mirror and recover their self-esteem12.
Another negative implication of facial lipoatrophy is its impact on treatment adherence, functioning as a disincentive. The facial, body, and lifestyle changes cause significant discomfort, leading many patients to abandon treatment15. The esthetic result of facial filling was quite satisfactory, and all of the patients were very satisfied or satisfied with the result obtained (Table 1).
Finally, besides living with HIV/AIDS, most patients are also affected by the various forms of lipoatrophy, visualized by reduction of adipose tissue in the face and consequent facial wrinkling, which confers an aspect of early aging or even fat loss of the buttocks, and upper and lower limbs, highlighting peripheral venous circulation.
Psychological repercussions have been described in patients who developed lipodystrophy, given that the syndrome has been reported by patients as a visible marker, identifying people with HIV infection.
The face, as an affected body segment, has been cited as an important stigmatizing factor for individuals with AIDS, making them more vulnerable to the identification of seropositivity, and consequently reflected in the degradation of patients' self-esteem and socialization.
Thus, the knowledge of the clinical and epidemiological profiles of this population can guide the multidisciplinary teams in programs and other official bodies in the field in the elaboration of more-effective strategies for the prevention and control of the disease, and rehabilitation of these patients.
The patients were predominantly male, Caucasian, and heterosexual, with a mean age of 49.7 years. On average, HIV was diagnosed 11.5 years prior, the duration of HAART use was 10 years, and facial lipoatrophy was diagnosed 3.8 years prior. Most of the patients used D4T and/or EVF. The mean CD4+ T lymphocyte count was 284 cells/mm3 at the beginning of the treatment and increased to 709 cells/mm3 during the course of the treatment. Plasma VL was undetectable in 88.9% of the patients. Dyslipidemia was present in 74.6% of the patients, of whom 50.8% used psychotropic drugs.
The answers provided by the patients indicated that the patients themselves noticed the change caused by the facial lipoatrophy in 74.6% of the cases. The patients themselves (76.2%) were more uncomfortable with the facial changes. In 85.7% of the cases, the patients did not reveal the diagnosis to third parties. The expectation of the result of the filling was high or very high in 79.4%, and all of the patients were satisfied or very satisfied with the result obtained. In all of cases, the impact was favorable and the adverse effects of filling were infrequent and transient.
CONCLUSION
Facial filling with PMMA is an alternative treatment to be used by plastic surgeons for facial lipoatrophy in AIDS patients. Moreover, most of the patients reported that they themselves perceived the facial lipoatrophy and that they were uncomfortable with the facial changes. Most of them also reported preferring not to reveal the diagnosis to third parties and that they had great expectations regarding the result of the facial filling.
The patients reported satisfaction with the outcome of facial filling with PMMA, which had a favorable impact on their lives.
COLLABORATIONS
WHM Analysis and/or data interpretation; statistical analysis; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and writing of the manuscript or critical review of its contents
KVOP Analysis and/or data interpretation; final approval of the manuscript; conception and design of the study; questionnaire; and writing of the manuscript or critical review of its contents
MAM Analysis and/or interpretation of data; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and writing of the manuscript or critical review of its contents
MHS Analysis and/or interpretation of data, and writing of the manuscript or critical review of its contents
GVPF Final approval of the manuscript, and conception and design of the study
LCA Statistical analysis and final approval of the manuscript
REFERENCES
1. Loyola AL, Corrêa M, Cassier M. Aids e saúde pública: a implantação de medicamentos genéricos no Brasil. In: Corrêa M, Cassier M, orgs. AIDS e saúde pública: contribuições à reflexão sobre uma nova economia política do medicamento no Brasil. Rio de Janeiro: EDUERJ; 2010. p.17-70.
2. Lima LAA. Evolução da terapia anti-retroviral para HIV/Aids - Linha do tempo. Atividade Científica. 2009;Sect. 3-21.
3. Pereira SBG, Gastaldi BS. Prevalência. In: Pereira SBG, ed. Lipodistrofia. 1a ed. São Paulo: Livraria Santos; 2007. p.9-11.
4. BRASIL. Lei nº 9.656, de 3 de junho de 1998. Dispõe sobre os planos e seguros privados de assistência à saúde. [Acesso 3 Dez 2012]. Disponível em: http://www.planalto.gov.br/ccivil_03/leis/L9656.htm
5. Lago RF, Costa NR. Dilemas da política de distribuição de medicamentos antirretrovirais no Brasil. Ciênc Saúde Coletiva. 2010;15(supl.3):3529-40. DOI: http://dx.doi.org/10.1590/S1413-81232010000900028
6. Lassalle S, Cervera P, Hofman V, Mari M, Dellamonica P, Hofman P. Antiretroviral treatments-related lipodystrophy syndrome: clinicopathological findings. Ann Pathol. 2005;25(4):309-17.
7. Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51-8. DOI: http://dx.doi.org/10.1097/00002030-199807000-00003
8. Han SH, Zhou J, Saghayam S, Vanar S, Phanuphak N, Chen YM, et al.; TREAT Asia HIV Observational Database. Prevalence of and risk factors for lipodystrophy among HIV-infected patients receiving combined antiretroviral treatment in the Asia-Pacific region: results from the TREAT Asia HIV Observational Database (TAHOD). Endocr J. 2011;58(6):475-84. DOI: http://dx.doi.org/10.1507/endocrj.K10E-407
9. Behrens G, Schmidt RE. Lipodystrophy syndrome. In: Hoffman C, Rockstroh JK, Kamps BS, eds. HIV Medicine 2006 [serial on the Internet]. [Acesso 17 Mai 2016]. Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/hivmedicine2006.pdf
10. Murray M. Lumpkin M. Reports of diabetes and hyperglycemia in patients receiving protease inhibitors for the treatment of human immunodeficiency virus (HIV). In: Bethesda: Food and Drug Administration; 1997.
11. Valente AM, Reis AF, Machado DM, Succi RC, Chacra AR. Metabolic alterations in HIV-associated lipodystrophy syndrome. Arq Bras Endocrinol Metab. 2005;49(6):871-81. DOI: http://dx.doi.org/10.1590/S0004-27302005000600004
12. Brasil. Ministério da Saúde. Tratamento da Lipoatrofia facial: Preenchimento com PMMA. In: Brasil. Ministério da Saúde do Brasil, Departamento de DST, Aids e Hepatites Virais. Manual de Tratamento da Lipoatrofia facial - Recomendações para o preenchimento facial com polimetilmetacrilato em portadores de HIV/AIDS. Brasília: Ministério da Saúde; 2009. p.27-37.
13. Luther J, Glesby MJ. Dermatologic adverse effects of antiretroviral therapy: recognition and management. Am J Clin Dermatol. 2007;8(4):221-33. DOI: http://dx.doi.org/10.2165/00128071-200708040-00004
14. Castelo Filho A, Abrão P. Alterações metabólicas do paciente infectado por HIV. Arq Bras Endocrinol Metab. 2007;51(1):5-7. DOI: http://dx.doi.org/10.1590/S0004-27302007000100003
15. Fuller J. A 39-year-old man with HIV-associated lipodystrophy. JAMA. 2008;300(9):1056-66. DOI: http://dx.doi.org/10.1001/jama.300.5.jrr80007
16. Alencar R, Caraciolo J, Fonsi M, Lotufo D, Yoshioka M. Lipodistrofia: desafio e soluções. BEPA Boletim Epidemiológico Paulista (Online). 2010;7:(74). [Acesso 17 Mai 2016]. Disponível em: http://www.cve.saude.sp.gov.br/agencia/bepa74_lipodistrofia.htm
17. Brasil. Ministério da Saúde. Departamento de DST, Aids e Hepatites Virais. Boletim Epidemiológico Aids e DST 2011. Brasília: Ministério da Saúde; 2011.
18. Loutfy MR, Raboud JM, Antoniou T, Kovacs C, Shen S, Halpenny R, et al. Immediate versus delayed polyalkylimide gel injections to correct facial lipoatrophy in HIV-positive patients. AIDS. 2007;21(9):1147-55. DOI: http://dx.doi.org/10.1097/QAD.0b013e3281c6148d
19. Brasil. Portaria nº 118 de 23 de fevereiro de 2005. Ministério da Saúde. 2005. [Acesso 17 Mai 2016]. Disponível em: http://www.brasilsus.com.br/legislacoes/sas/4084-118
20. Dean AG, Dean JA, Coloumbier D, Brendel KA, Smith DC, Burton AH, et al. Epi Info version 6.0: a word processing database and statistics program for epidemiology on microcomputers. Atlanta: Centers for Disease Control and Prevention; 1994.
21. Ribera E, Paradiñeiro JC, Curran A, Sauleda S, García-Arumí E, Castella E, et al. Improvements in subcutaneous fat, lipid profile, and parameters of mitochondrial toxicity in patients with peripheral lipoatrophy when stavudine is switched to tenofovir (LIPOTEST study). HIV Clin Trials. 2008;9(6):407-17. DOI: http://dx.doi.org/10.1310/hct0906-407
22. Abood A, Ong J, Withey S, Johnson M, Butler P. Facial atrophy in HIV-related fat redistribution syndrome: a plastic surgical perspective on treatment options and a look to the future. Int J STD AIDS. 2006;17(4):217-20. DOI: http://dx.doi.org/10.1258/095646206776253345
23. Martinez TLR. Alterações metabólicas presentes na infecção pelo HIV - revisão atualizada da sua etiopatogenia e tratamento. Rev Atividade Científica. 2008;3(29):3-1815.
24. Paton NI, Earnest A, Ng YM, Karim F, Aboulhab J. Lipodystrophy in a cohort of human immunodeficiency virus-infected Asian patients: prevalence, associated factors, and psychological impact. Clin Infect Dis. 2002;35(10):1244-9. DOI: http://dx.doi.org/10.1086/34405
25. Peterson S, Martins CR, Cofrancesco J Jr. Lipodystrophy in the patient with HIV: social, psychological, and treatment considerations. Aesthet Surg J. 2008;28(4):443-51. DOI: http://dx.doi.org/10.1016/j.asj.2008.04.009
26. Hornberger J, Rajagopalan R, Shewade A, Loutfy MR. Cost consequences of HIV-associated lipoatrophy. AIDS Care. 2009;21(5):664-71. DOI: http://dx.doi.org/10.1080/09540120802511851
27. Portilla J. Tenofovir as a strategy to avoid or limit adverse effects. Enferm Infecc Microbiol Clin. 2008;26 Suppl 8:19-24. PMID: 19195434
28. Crane HM, Grunfeld C, Harrington RD, Uldall KK, Ciechanowski PS, Kitahata MM. Lipoatrophy among HIV-infected patients is associated with higher levels of depression than lipohypertrophy. HIV Med. 2008;9(9):780-6. DOI: http://dx.doi.org/10.1111/j.1468-1293.2008.00631.x
29. Hirsch HH, Battegay M. HIV-infection, HAART (highly-active antiretroviral therapy) and hyperlipidemia. Dtsch Med Wochenschr. 2003;128(19):1051-4. DOI: http://dx.doi.org/10.1055/s-2003-39100
30. Bechara FG, Sand M, Potthoff A, Altmeyer P, Brockmeyer NH; German Competence Network for HIV/AIDS. HIV-associated facial lipoatrophy--review of current therapy options. Eur J Med Res. 2008;13(3):93-9.
31. Jones D. HIV facial lipoatrophy: causes and treatment options. Dermatol Surg. 2005;31(11 Pt 2):1519-29. DOI: http://dx.doi.org/10.2310/6350.2005.31237
32. Matos AC, Boletini RS, Kating TC, Matsumoto NF, Gandolpho MA. Tratamento da lipoatrofia facial em pessoas vivendo com HIV/AIDS: afastando o preconceito e melhorando a qualidade de vida. Mundo Saúde. 2010;34(2):210-7.
33. Salles AG, Lotierzo PH, Gemperli R, Besteiro JM, Ishida LC, Gimenez RP, et al. Complications after polymethylmethacrylate injections: report of 32 cases. Plast Reconstr Surg. 2008;121(5):1811-20. PMID: 18454007
34. Niamtu J 3rd. Filler injection with micro-cannula instead of needles. Dermatol Surg. 2009;35(12):2005-8. DOI: http://dx.doi.org/10.1111/j.1524-4725.2009.01323.x
35. Rogowska-Szadkowska D, Chlabicz S, Oltarzewska MA, Sawicka-Powierza J. Which factors hinder the decision of Polish HIV-positive patients to take up antiretroviral therapy? AIDS Care. 2009;21(3):280-3.
1. Faculdade de Medicina do ABC, Santo André, SP, Brazil
2. Programa Municipal de DST/AIDS e Hepatites Virais de São Bernardo do Campo, SP, Brazil
3. Harvard T.H. Chan School of Public Health, Boston, Estados Unidos
Institution: Ambulatório de Lipodistrofia do Programa Municipal de DST/AIDS e Hepatites Virais de São Bernardo do Campo.
Corresponding author:
Walter Henrique Martins
Av. Angélica, 1757, 1º andar - Higienópólis
São Paulo, SP, Brazil Zip Code 01227-200
E-mail: whenriquemartins@uol.com.br
Article received: July 25, 2013.
Article accepted: February 4, 2014.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter