

Original Article - Year 2016 - Volume 31 -
Reduction mastoplasty with submuscular implants for breast symmetrization in patients undergoing mastectomy
Mastoplastia redutora com implante submuscular para simetrização mamária em pacientes submetidas à mastectomia
ABSTRACT
INTRODUCTION: Breast reconstruction with expanders/implants is one of the most common techniques used for the treatment of mastectomy-induced sequelae, due to the reduced surgical time and morbidity. However, the maintenance of long-term symmetrization in the contralateral breast remains a major challenge. The procedure of reduction mastoplasty has been developed, and is performed by positioning a submuscular implant in the contralateral breast of patients undergoing breast reconstruction.
METHODS: A total of 31 patients were included in the study. Their primary characteristics were glandular resection, combined with implant insertion in the subpectoral plane. Complications, shape, symmetry, and volume were subsequently evaluated.
RESULTS: A low incidence of complications and surgical revisions was observed, with no cases of implant loss. Good breast symmetry was achieved.
CONCLUSION: Reduction mastoplasty with submuscular implants proved to be a safe procedure, and resulted in good breast symmetry in patients who underwent mastectomy.
Keywords: Breast neoplasms; Mammoplasty; Breast implant; Mastectomy.
RESUMO
INTRODUÇÃO: A reconstrução mamária com uso de expansores/implantes é uma das técnicas mais empregadas para tratamento das sequelas de mastectomia devido ao menor tempo cirúrgico e menor morbidade. No entanto, a manutenção da simetria com a mama contralateral a longo prazo continua a ser um grande desafio. É proposta, então, técnica de mastoplastia redutora com colocação de implante submuscular na mama contralateral de pacientes com reconstrução mamária.
MÉTODOS: Foram incluídas 31 pacientes submetidas a esta técnica, cujas características principais são ressecção glandular associada à inclusão de prótese em plano subpeitoral total. Foram avaliadas as complicações e simetria de forma e volume obtida.
RESULTADOS: Observou-se baixa incidência de complicações e revisões cirúrgicas, nenhum caso de perda do implante e bons resultados de simetria.
CONCLUSÃO: A mastoplastia redutora com implante submuscular mostrou-se técnica segura, com bons resultados de simetria em pacientes mastectomizadas.
Palavras-chave: Neoplasias da mama; Mamoplastia; Implante mamário; Mastectomia.
The incidence of breast cancer, the most common cancer in women, is increasing1. About 12% of women will develop breast cancer during their lives. Surgical treatment based on breast removal (mastectomy) remains the most suitable approach2.
Breast reconstruction with an expander and subsequent exchange for implants is one of the most commonly used techniques for the treatment of mastectomy-induced sequelae. This is due to the reduced surgical time and morbidity, combined with improvement in the quality of the implants. However, maintaining long-term shape symmetry and consistency between reconstructed and contralateral breasts, especially if the latter is large and ptotic, remains a major challenge.
Usually, reduction mastoplasty or mastopexy is carried out in the contralateral non-mastectomized breast to achieve symmetry3,4. However, the projection of the upper pole of the breast without implants almost always remains less than that of the projection of the breast reconstructed with implants. With aging, there is even more asymmetry, as fat replacement and skin sagging occur almost exclusively in non-mastectomized breasts.
With the aim of improving symmetry, several authors began to use mastopexy combined with the placement of subglandular implants in the contralateral breast. Despite the improvement in consistency and upper pole projection, adding implant weight to an ongoing aging process results in breast asymmetry in the long term, which subsequently requires surgical revision.
An alternative capable of providing more satisfactory long-term results and improved breast symmetry is reduction mastoplasty with a submuscular implant.
OBJECTIVE
The aim of this study is to evaluate breast symmetry after reduction mastoplasty with a submuscular implant in patients undergoing contralateral mastectomy.
METHODS
This primary retrospective study analyzed the medical records and photographic information of patients who underwent reduction mastoplasty with a submuscular implant to achieve breast symmetry. The procedures evaluated were performed between 2009 and 2013 by the first author of this study at the Women's Health Referral Center - Pérola Byington Hospital, São Paulo, SP.
Medical records were reviewed and information on demographics, initial illness, comorbidities, adjunctive treatment, surgeries, and complications were collected. Pre-and postoperative photographs were independently analyzed by 2 plastic surgeons with experience in breast reconstruction. The postoperative photographs were taken 6-14 months after breast symmetrization. The 2 plastic surgeons were asked to assign individual scores of 0 to 10 for volume (size) symmetry, shape symmetry, and the general appearance of the breast that underwent reduction mastoplasty with a submuscular implant. The study was approved by the Research Ethics Committee of the institution as number 664.599.
All patients underwent preoperative assessment, including breast ultrasound and mammography, and were subsequently discharged by a breast specialist. The resected tissue was sent for anatomopathological examination. All patients had general anesthesia and received a first-generation cephalosporin for 7 days.
Inclusion Criteria
Patients underwent reduction mastoplasty with a submuscular implant to achieve breast symmetry following modified radical mastectomy between 2009 and 2013 by the first author of this study, at the Women's Health Referral Center - Pérola Byington Hospital.
Cohort
Thirty-one patients were included in the study. Of these, 28 underwent breast reconstruction with an expander and 3 received a latissimus dorsi flap in combination with an expander.
Implant choice
When reduction mastoplasty with a submuscular implant is performed in combination with exchange of the expander in the mastectomized breast, the implant for the mastectomized breast is chosen first. The volume injected into the expander and the desired size of the breast base (linear measurement in frontal view) are taken into account, with limits defined by the anterior axillary line and the lateral edge of the sternum. The projection is also measured. The implant is chosen by using a table defining dimensions and volumes ensured by the manufacturer. In general, a textured, round, high-projection implant is used, with a slightly lower (20 to 50 ml) volume than the volume used in the expander.
Then, the contralateral breast implant is chosen. The base of the existing breast is measured (frontal linear measurement, from the medial to the lateral margin of the breast). If the breast extends laterally to the anterior axillary line, the measurement should extend to this line (Figure 1). Then, 2-4 cm are subtracted from this measurement, based on the amount of existing parenchyma. In breasts with little parenchyma, less is subtracted, and vice versa (Figure 2). Using a table defining dimensions and volumes ensured by the manufacturer, the implant for the mastectomized breast is chosen. Usually, a textured, round. high- or moderate-projection implant is used.
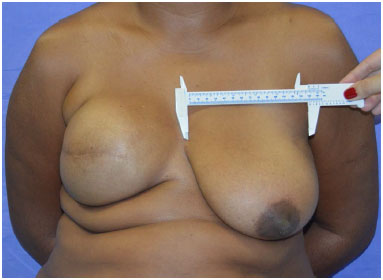
Figure 1. Measurement of the base of the breast, without exceeding the anterior axillary line.
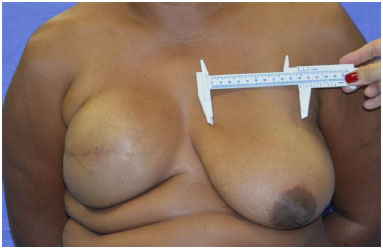
Figure 2. 2 to 4 cm are subtracted from the base of the breast to select the base of the implant.
Marking
With the patient in supine position, the midline and line corresponding to the inframammary fold are drawn. In the mastectomized breast, the best position for areolar reconstruction is used, corresponding to the central area of the cone (Figure 3).
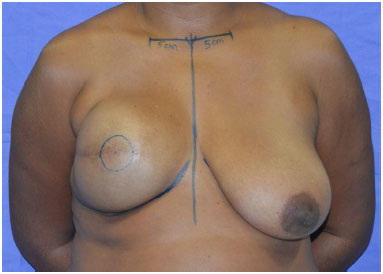
Figure 3. Marking the position of the future areola on the breast cone apex.
The breast axes are then traced with a line 5 cm from the sternal notch to the clavicle, and then extending toward the papilla. If the axes have a very divergent angle due to increased laterality of the healthy breast, a new mirror line is traced on the healthy breast to match the axis of the mastectomized breast. Using a horizontal ruler, the upper point of the areola in the mastectomized breast is transferred to the healthy breast (point "A") (Figure 4).
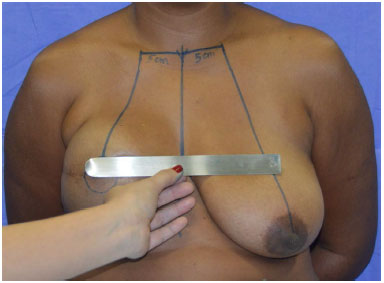
Figure 4. After drawing breast axes, the position of the areola is transferred to the contralateral breast (point A).
The distance from point "A" to the inframammary fold (pillar) of the mastectomized breast is then measured.
Using a clamping maneuver in the healthy breast, 2 lines are drawn to form a triangle, starting from point "A", with lengths equal to the pillar of the other breast (Figure 5). From the inferior points of these lines (named "B" and "C"), the marking extends vertically toward the inframammary fold (Figure 6).
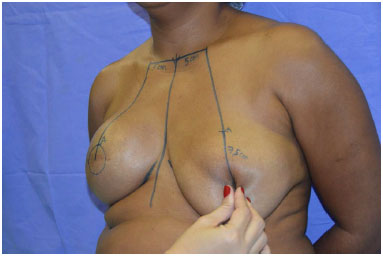
Figure 5. Clamping maneuver to mark the edges of the triangle.
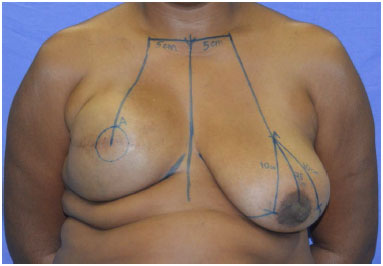
Figure 6. Final marking.
Surgical technique
When the surgical time combines the exchange of the expander in the mastectomized breast, the procedure is first performed through a scar left by the prior mastectomy. Then, contralateral mastoplasty is performed, starting with the removal of periareolar epidermis and the pedicle area of the nipple-areolar complex, using the prior marking. A careful incision is carried out in the inframammary fold, preserving the superficial pectoral fascia and part of the subcutaneous tissue in the inframammary fold region (Figure 7).

Figure 7. Incision in the inframammary fold preserving the pectoral fascia.
Using monopolar electrocautery, the procedure continues with suprafascial detachment toward the areola. The inferior pole of the breast is then removed (Figure 8).
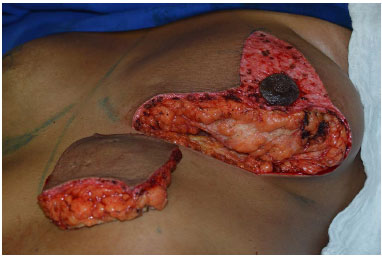
Figure 8. Resection of the lower pole of the breast.
Once the lateral margin of the pectoralis major muscle is identified, the subpectoral region is dissected under direct vision, with the aid of an illuminated retractor and using monopolar electrocautery (Figure 9).
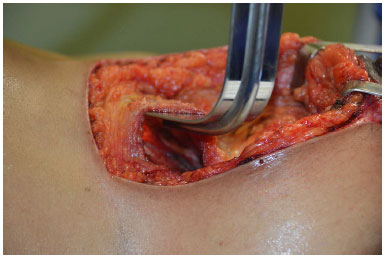
Figure 9. Subpectoral plane dissection.
The inferior margin of the pectoralis major muscle is elevated along with the superficial pectoral fascia in the inframammary fold, exceeding the fold by 1 cm. The medial lower insertions of the pectoral muscle near the sternum are divided from the 4th intercostal space toward the inframammary fold (Figure 10).
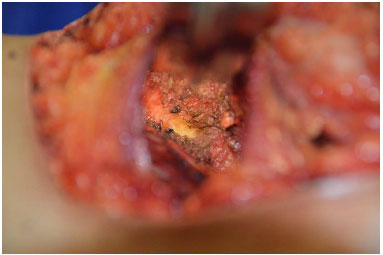
Figure 10. Medial detachment of the pectoralis muscle between the 4th intercostal space and the inframammary fold.
The nipple-areolar complex is elevated through the superior or superomedial pedicle and positioned in the previously marked location. The region is then washed with saline solution and homeostasis revised. A vacuum tubular drain is applied and drainage is performed in case of exacerbated intraoperative bleeding. The selected implant is then soaked in saline solution (100 ml) with 80 mg of gentamicin and 1 g of cephalothin, and positioned in the subpectoral region (Figure 11).
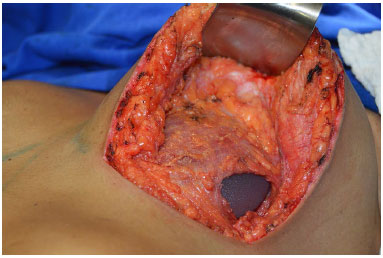
Figure 11. Implant positioning in the subpectoral plane.
Approximation of the glandular tissue of the lateral portion of the breast to the lateral margin of the pectoralis major muscle is carried out with Vicryl 2-0, to completely cover the implant (Figure 12).
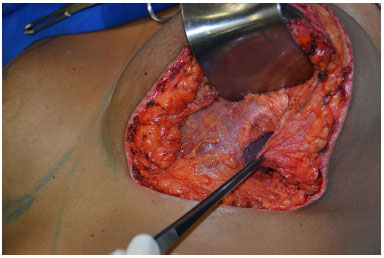
Figure 12. Approximation of the glandular tissue alongside the pectoral muscle to suture it and cover the implant.
If necessary, reduction of the retroareolar glandular tissue and of the lateral and medial pillars of the breast is carried out for volume adjustment. The patient is in a sitting position to ensure better symmetry assessment (Figure 13).
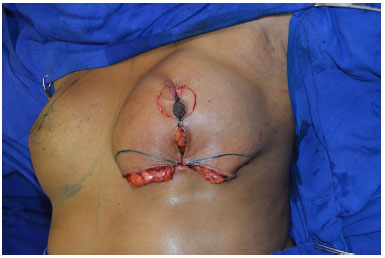
Figure 13. The patient is sitting and the area for the resection of the medial and lateral excess is marked.
Suturing is performed with nylon 2-0 at the junction of the vertical and horizontal scars (inverted "T" junction), followed by nylon 3-0 in the glandular tissue. Subdermal sutures are placed with nylon 4-0, and intradermal sutures with Monocryl 4-0 (Figure 14).
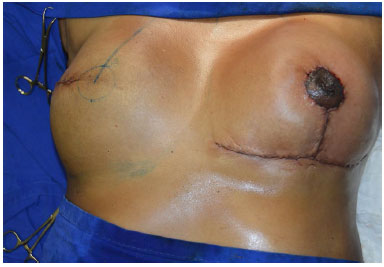
Figure 14. End of the surgery.
RESULTS
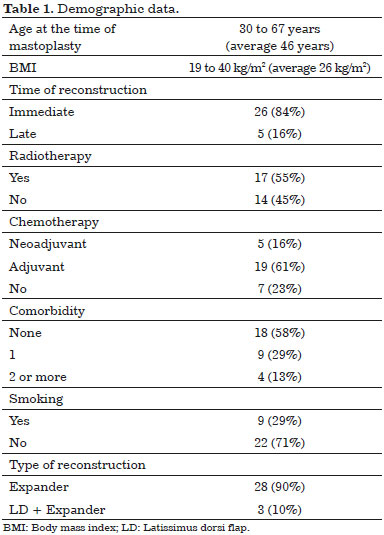
Twenty-six patients (84%) underwent immediate reconstruction (at the time of mastectomy) and 5 (16%) had late reconstruction.
Eighteen patients (58%) had no comorbidities, while 9 (29%) had 1 comorbidity (7 with hypertension and dyslipidemia and 1 with asthma). Four patients (13%) had 2 or more comorbidities (1 with hypertension/hypothyroidism/mitral valve prolapse, 1 with hypertension/type 2 diabetes mellitus, 1 with hypertension/mitral insufficiency, and 1 with hypertension/dyslipidemia). Nine patients (29%) smoked in the year prior to reconstruction.
The most prevalent type of tumor was ductal carcinoma; 22 patients had invasive ductal carcinoma (71%) and 4 had in situ ductal carcinoma (13%).
Five patients (16%) received neoadjuvant chemotherapy before mastectomy and 19 (61%) had adjuvant chemotherapy after mastectomy, before implant replacement and symmetrization. Seventeen patients (55%) received radiotherapy after mastectomy and before implant exchange surgery.
Table 1 summarizes the main characteristics of the patients included in the study.
The patients who underwent reconstruction with a single expander received implants measuring 300-575 ml (average 470 ml) in the mastectomized breast. Of these, 22 received a round, high profile, 3 a round, moderate profile, and 1 a round, low-profile implant, and 2 received anatomical implants. Three patients in whom the latissimus dorsi was combined with the expander received a 350-ml round, high-profile implant, a 450-ml round, moderate profile implant, and a 440-ml round, high-profile implant, respectively.
Eight patients (26%) had undergone prior mastoplasty in the contralateral healthy breast (secondary surgery); 7 underwent breast symmetrization after mastectomy, and 1 had undergone bilateral reduction mastoplasty 20 years before.
Of 31 patients, 5 (16%) had breast ptosis grade I according to the Regnault classification5, 13 (42%) had grade II, 10 (32%) had grade III, and 3 (10%) had pseudoptosis.
In 21 of 31 patients, the weight of the tissue removed during reduction mastoplasty with a submuscular implant was measured. The average resected tissue weighed 165 g (range, 58 g to 350 g). All specimens were sent for anatomopathological examination, but none showed signs of malignancy. The implants that were placed varied between 160 ml and 350 ml (average 202 ml). These were all texturized and round - 20 with high profile, 2 with moderate profile, and 9 with low profile.
All but 1 patient was discharged the day after surgery. A vacuum tubular drain (Portovac 4.8) was placed in 1 patient.
The average follow-up period was 2 years and 10 months (range, 6 months to 5 years). The data are summarized in Table 2.
Complications are summarized in Table 3.
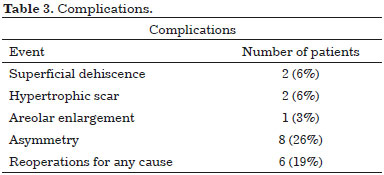
No patient developed a hematoma or seroma. Two developed a small superficial dehiscence, which resolved with clinical treatment. Two patients developed hypertrophic scars, and were treated with silicone gel sheeting with good results. One patient developed areolar enlargement and underwent outpatient correction.
Eight patients had significant breast asymmetry during the follow-up period, and were offered surgical revision. Two did not want to undergo additional surgery, and were satisfied with the reconstruction. Five underwent additional surgery. One patient who developed recurrent ptosis of the non-mastectomized breast 4 years after surgery had already been scheduled for further surgery. All additional surgeries are summarized in Table 4.
Two patients who received radiotherapy presented with implant elevation in the mastectomized breast, and had surgical correction. Since the complication was not in the breast with the reduction mastoplasty with submuscular implant, they were not included in the list of complications. Likewise, three patients who developed implant extrusion of the mastectomized breast and underwent further reconstruction using the latissimus dorsi and implant were not included in the list of complications (Figure 15). Breast cancer recurred in 1 patient, who underwent extended resection and implant removal by a breast surgeon 3 years after symmetrization.
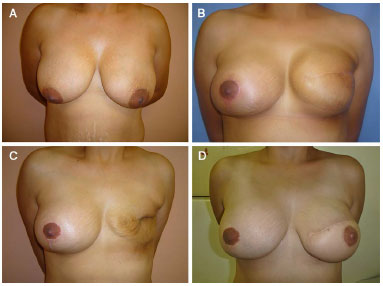
Figure 15. A: A 31-year old patient underwent mastectomy and immediate expander positioning in 2009. B: Two months after exchange for a 500-ml high-profile implant and symmetrization with reduction mastoplasty, using a 200-ml high-profile submuscular implant. C: The patient developed extrusion and underwent removal of the implant from the mastectomized breast. D: Nine months after a new reconstruction (latissimus dorsi + 340 high profile implant), and 2 months after nipple-areolar reconstruction (graft).
Volume symmetry received a better average score in the photographic evaluation (8.81, standard deviation 1.62) by the 2 evaluators. Shape symmetry received an average score of 7.16 (standard deviation 1.99). The average score for the general appearance of a breast that underwent reduction mastoplasty with a submuscular implant was 8.75 (standard deviation 1.40). The averages are shown in Figure 16.
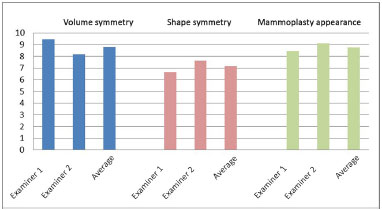
Figure 16. Photographic evaluation of the patients: scores for volume symmetry, shape symmetry, and individual appearance of reduction mastoplasty with submuscular implant.
DISCUSSION
Postmastectomy breast reconstruction with expanders and implants is considered the technique of choice by several authors. This is based on known characteristics, such as reduced surgical time and absence of morbidity in the donor area. However, techniques for breast symmetrization are rarely cited. Conventional reduction mastoplasty or mastopexy with subglandular implants provides good symmetry for a very short duration. The sagging of the non-mastectomized breast causes loss of projection of the upper pole in the medium and long term. Even patients who underwent breast reconstruction with the latissimus dorsi flap combined with expanders or implants often encountered difficulty in performance of the symmetrization procedure. Therefore, these patients were included in the study.
In 2013, Sampaio et al.6 published a technique named structured mammoplasty for breast symmetrization. The authors describe resection of the Chassaignac bursa to reduce breast mobility on the thorax, and placed an implant in the partial subpectoral plane ("dual plane"). This partial subpectoral plane is characterized by the detachment of the pectoralis major muscle inferiorly, thus leaving the implant without muscle coverage in its lower part6,7.
Therefore, there is no weight support for the muscle implant and an additional coverage plane in case of dehiscence. Considering the high prevalence of comorbidities in patients who are candidates for this surgery and the possible use of adjuvant chemo- and radiation therapy, wound dehiscence and implant exposure might occur. However, in the technique described in this study, the plane used to insert the implant is total subpectoral (without detachment of the pectoralis major muscle inferiorly). There is more coverage of the implant and better fixation of its position8,9.
Daher et al.8, in 2012, reported that mastopexy can be performed with a submuscular implant for the aesthetic correction of the breast. These authors also used the total subpectoral plane to better support the implant. However, a central incision was performed in the pectoralis major muscle to position the implant, and it often remains open. In the technique described herein, the implant is placed through the lateral margin of the muscle, which is elevated but not cut. This margin is then sutured to the remaining lateral part of the gland, thus completely covering the implant.
Daher et al.8 also released the medial insertions of the pectoralis major muscle between the 4th intercostal space and the inframammary fold. This maneuver reduces muscle action, which might result in the elevation of the implant. The authors also recommend performing glandular resection in large and heavy breasts, which prevents the gland from sliding over the implant and the occurrence of ptosis, pseudoptosis, or a "double breast" effect. Of 94 patients followed up for 1 year, 7 developed pseudoptosis. While 4 were mild, 3 required further surgery.
Beale et al.10, in 2014, reviewed 83 patients who underwent mastopexy with subpectoral implants to treat breast ptosis. However, patients who needed elevation of the areola more than 4 cm were excluded from this study, and underwent a 2-stage procedure. In this technique, the position of the areola is marked in the inframammary fold projection. The pedicle used is always inferior, and there is no medial detachment of the pectoralis muscle. Such differences could possibly interfere with breast symmetry in mastectomized patients. This is due to the peculiarities presented by the breast reconstructed with an expander/implant, such as an elevated position of the areola and a more constricted inferior pole.
Even with a cohort of patients with a range of ages, including the elderly, and with a high prevalence of overweight/obesity, comorbidities, and smoking, the incidence of complications in this study was quite low. No implant loss was observed, probably because the pectoralis muscle covered the implant and reduced the risk of extrusion and infection.
Good scarring results, with only 1 areolar enlargement and 2 hypertrophic scars, could also be related to the muscular support of the implant, which reduces the stress transmitted to the skin. In combination with a decreased glandular volume, this seems to reduce ptosis recurrence and the need for surgical revision. Of only 6 additional surgeries performed during the follow-up period, 3 involved cases treated within the first few months of the study.
The photographic assessment scores by the 2 evaluators were quite high, indicating good quality of symmetry. The scores for the appearance of the individual breasts following reduction mastopexy with submuscular implants were excellent. Due to the small cohort, it was not possible to statistically compare groups of patients, such as those who received radiotherapy, or who were smokers or non-smokers.
McCarthy et al.11, in 2008, retrospectively evaluated 1,170 breast reconstructions using an expander/implant and concluded that smoking, obesity, hypertension, and age above 65 years are independent risk factors for complications. Kronowitz and Robb12, in a review of the literature published in 2009, observed that patients who received radiotherapy had the lowest scores for symmetry.
Future prospective studies with a larger cohort are needed to validate the effectiveness of the technique. Assessment of the quality of life, self-esteem, and patient satisfaction could also show different results between symmetrization procedures.
Yueh et al.13, in 2010, reported decreased patient satisfaction following breast reconstruction with expanders/implants, when compared to patients in whom autologous tissue was used to perform the same procedure Decreased patient satisfaction was observed when breast reconstruction was performed with the latissimus dorsi compared to the use of abdominal tissue. However, the authors did not describe contralateral breast symmetrization surgery. Therefore, the great difficulty in obtaining symmetry in patients with implants might have negatively influenced the results.
Moreover, patients with a history of breast cancer have an increased risk for cancer in the opposite breast. Therefore, symmetrization surgery should not interfere with early diagnosis and should consider the possibility of a future mastectomy (Figure 17). In the technique presented herein, the non-violation of the pectoral fascia maintains a natural barrier against deep invasion by tumors, and does not prevent future reconstruction with an expander. Glandular breast reduction reduces surgical risk by providing large samples of tissue for histopathological analysis14.
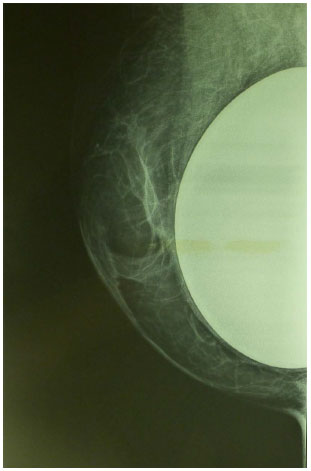
Figure 17. Mammograms of breasts that underwent reduction mastoplasty with submuscular implants for breast symmetrization. Note the good visualization of the remaining glandular tissue and the optimal positioning of the implant.
Breast symmetrization surgery after mastectomy is challenging. Specific characteristics of the reconstructed breast, such as projection, position of the areola, and consistency are difficult to mimic in the opposite breast. Reduction mastoplasty with a submuscular implant was shown to be a safe technique, and is capable of providing good results. Therefore, we encourage its use.
CONCLUSION
Reduction mastoplasty with a submuscular implant proved to be a safe procedure, with a low complication rate and good symmetry results in mastectomized patients.
COLLABORATIONS
LFF Analysis and/or interpretation of the data; statistical analysis; final approval of the manuscript; formulation of the hypotheses and study design; performance of the surgeries and/or experiments; manuscript preparation and critical review of the content.
ACBB Analysis and/or interpretation of the data; performance of the surgeries and/or experiments.
JB Analysis and/or interpretation of the data; statistical analysis; performance of the surgeries and/or experiments.
LEFA Formulation of the hypotheses and study design; performance of the surgeries and/or experiments; manuscript preparation and critical review of the content.
LHG Formulation of the hypotheses and study design; manuscript preparation and critical review of the content.
REFERENCES
1. Instituto Nacional de Câncer José Alencar Gomes da Silva (Brasil). Estimativa 2014. Incidência de Câncer no Brasil. Coordenação de Prevenção e Vigilância. Rio de Janeiro: INCA; 2014. 124p.
2. American Cancer Society. What are the key statistics about breast cancer? [Acesso 26 Jul 2014]. Disponível em http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics
3. Losken A, Carlson GW, Bostwick J 3rd, Jones GE, Culbertson JH, Schoemann M. Trends in unilateral breast reconstruction and management of the contralateral breast: the Emory experience. Plast Reconstr Surg. 2002;110(1):89-97. PMID: 12087236 DOI: http://dx.doi.org/10.1097/00006534-200207000-00016
4. Leone MS, Priano V, Franchelli S, Puggioni V, Merlo DF, Mannucci M, et al. Factors affecting symmetrization of the contralateral breast: a 7-year unilateral postmastectomy breast reconstruction experience. Aesthetic Plast Surg. 2011;35(4):446-51. DOI: http://dx.doi.org/10.1007/s00266-010-9622-7
5. Regnault P. Breast ptosis. Definition and treatment. Clin Plast Surg. 1976;3(2):193-203.
6. Sampaio MM, Fraga M, Ferreira AP, Barros AC. Structured mammaplasty: a new approach for obtaining breast symmetry. Plast Reconstr Surg. 2013;131(2):300e-302e. PMID: 23358041 DOI: http://dx.doi.org/10.1097/PRS.0b013e318278d7d9
7. Tebbetts JB. Dual plane breast augmentation: optimizing implant-soft-tissue relationships in a wide range of breast types. Plast Reconstr Surg. 2006;118(7 Suppl):81S-98S; discussion 9S-102S. DOI: http://dx.doi.org/10.1097/00006534-200612001-00012
8. Daher JC, Amaral JDLG, Pedroso DB, Cintra Júnior R, Borgatto MS. Mastopexia associada a implante de silicone submuscular ou subglandular: sistematização das escolhas e dificuldades. Rev Bras Cir Plást. 2012;27(2):294-300. DOI: http://dx.doi.org/10.1590/S1983-51752012000200021
9. Garcia EB, Fusaro Neto R, Arruda RF, Pereira JB, Ferreira LM. Inferior pedicle breast flap for submuscular implant coverage in mammaplasty after massive weight loss. Plast Reconstr Surg. 2010;125(2):74e-75e. PMID: 20124815 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181c72592
10. Beale EW, Ramanadham S, Harrison B, Rasko Y, Armijo B, Rohrich RJ. Achieving predictability in augmentation mastopexy. Plast Reconstr Surg. 2014;133(3):284e-292e. PMID: 24572873 DOI: http://dx.doi.org/10.1097/PRS.0000000000000079
11. McCarthy CM, Mehrara BJ, Riedel E, Davidge K, Hinson A, Disa JJ, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121(6):1886-92. PMID: 18520873 DOI: http://dx.doi.org/10.1097/PRS.0b013e31817151c4
12. Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124(2):395-408. PMID: 19644254 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181aee987
13. Yueh JH, Slavin SA, Adesiyun T, Nyame TT, Gautam S, Morris DJ, et al. Patient satisfaction in postmastectomy breast reconstruction: a comparative evaluation of DIEP, TRAM, latissimus flap, and implant techniques. Plast Reconstr Surg. 2010;125(6):1585-95. PMID: 20517080 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181cb6351
14. Tarone RE, Lipworth L, Young VL, McLaughlin JK. Breast reduction surgery and breast cancer risk: does reduction mammaplasty have a role in primary prevention strategies for women at high risk of breast cancer? Plast Reconstr Surg. 2004;113(7):2104-10; discussion 11-2. DOI: http://dx.doi.org/10.1097/01.PRS.0000122407.07002.95
1. Centro de Referência da Saúde da Mulher, Hospital Pérola Byington, São Paulo, SP, Brazil
2. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
Institution: Centro de Referência da Saúde da Mulher - Hospital Pérola Byington.
Corresponding author:
Lia Fleissig Ferreira
Av. Brigadeiro Luís Antônio, 683 - Bela Vista
São Paulo, SP, Brazil Zip Code 01317-000
E-mail: liafleissig@gmail.com
Article received: August 6, 2014.
Article accepted: February 29, 2016.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter