

Case Report - Year 2015 - Volume 30 -
Acute presentation of Mondor's disease after breast augmentation with silicone implants
Apresentação aguda da doença de Mondor em pós-operatório de mamoplastia de aumento com implantes de silicone
ABSTRACT
The first breast augmentation surgery with silicone implants was performed at the Jefferson Davis Hospital in Houston (USA) about 50 years ago. Recent advances in medical technology have made implants of various shapes and textures commercially available and led to the development of numerous techniques for performing this surgery. However, this surgical procedure may have some immediate and long-term local complications . Since the implant is made of biocompatible material , it is important to investigate and report complications that occur despite the 50 years of research. The purpose of this study was to review the most frequent complications occurring after breast augmentation surgery with silicone implants and to report a case of an unusual complication, Mondor's disease.
Keywords: Mammoplasty; Breast implant; Skin abnormalities.
RESUMO
Há 50 anos, no Jefferson Davis Hospital, em Houston (EUA), realizou-se a primeira cirurgia de mamoplastia de aumento com implantes de silicone. Atualmente, o avanço da tecnologia médica disponibilizou no mercado implantes de diversas formas e texturas, assim como permitiu o desenvolvimento de inúmeras técnicas para a realização desta cirurgia. Este procedimento cirúrgico pode apresentar algumas complicações locais imediatas e tardias no pós-operatório. Por se tratar de um implante constituído de material biocompatível ao organismo, mesmo com 50 anos de evolução, deve-se sempre estudar e, se possível, relatar as possíveis complicações que possam ocorrer. O objetivo deste artigo é revisar as complicações mais frequentes que ocorrem no pós-operatório das mamoplastias de aumento com implante de silicone, bem como relatar o caso de uma complicação atípica, doença de Mondor, no pós-operatório desta cirurgia.
Palavras-chave: Mamoplastia; Implante mamário; Anormalidades cutâneas.
In 1962, Timmie Jean Lindsey underwent the first breast augmentation surgery with silicone implants at the Jefferson Davis Hospital in Houston (USA). This implant was designed and implanted by Thomas Cronin and Frank Gerow and produced by Dow Corning1. Since then, a variety of implants have become commercially available, and a multitude of techniques have been developed to perform this type of surgery. The idea of Cronin and Gerow has become popular, and today breast augmentation is the most frequently performed cosmetic surgery in Brazil and the United States of America (USA)2,3.
This surgical procedure may have some immediate and long-term local adverse effects, of which bruising, contracture, implant extrusion, infection of surgical wound, asymmetry, displacement, and stretch marks on the skin are the most frequent. One of the unusual complications is Mondor's disease4-8.
Mondor's disease is a thrombophlebitis of superficial breast veins, such as the thoracoepigastric vein and/or its tributaries. It is a rare and self-limited entity of idiopathic etiology. It is characterized clinically by the presence of injury in the form of a superficial fibrous cord corresponding to a hampered9,10 venous path.
Since implants are designed to be biocompatible, such cases should be carefully studied and presented even after 50 years of research and development.
The purpose of this article is to review the most frequent adverse effects of breast augmentation with silicone implants and report a case of an unusual and unknown complication.
CASE REPORT
A 28-year-old white woman underwent implantation of 255-ml silicone breast implants with polyurethane cover in the retro-glandular position through an incision in the inframammary crease. Thirty days after the surgery, after a sudden movement, the patient felt that the skin was tearing, which occurred not in the incision area or the breasts but in the right upper quadrant of the abdomen. A longitudinal fibrous superficial lump formed approximately 20 cm below the inframammary crease incision on the right side (Figure 1). There were no signs of inflammation or skin pain.
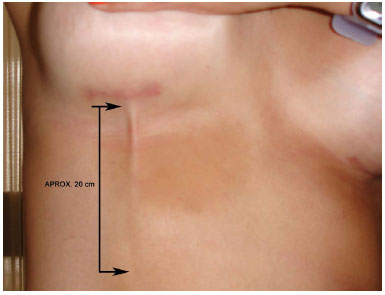
Figura 1. Detail of the fold formed in the skin.
DISCUSSION
Despite the 50 years of experience, advances in the breast silicone implants technology, and availability of numerous surgical techniques, complications are not uncommon. Although the morbidity is low, such complications invariably cause substantial physical and psychological discomfort.
The most frequent complications are listed in Table 1. In addition, less frequent adverse effects include, among others, symmastia caused by medial displacement of the implant, extrusions, early breaks, hypertrophic scars, and stretch marks4-10.
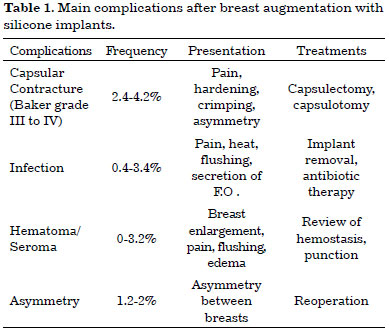
The most frequent complications are capsular contractures of grades III and IV (Baker classification)4-10. It is believed that the intensity of the inflammatory response determines the onset of these complications. Besides these known adverse effects, the immune response can cause other, unknown complications. It is important to report such individual cases given that breast augmentation is the most often performed plastic surgery in USA and Brazil2,3.
Mondor's disease, described in 1939 by Henri Mondor, occurs in approximately 0.48% to 1.07% of cases of breast augmentation surgery with silicone implants. Usually it appears during the third week after surgery, is self-limited, and may persist for 2-8 weeks. It can be accompanied with inflammation and pain, and non-steroidal anti-inflammatory drugs and analgesics may be used in such cases. Anticoagulants and antibiotics are not suitable9,10.
In the case of our patient, the development was favorable, and the abruptly formed deformity (superficial fibrous cord) was resolved after massaging the area with moisturizing creams, becoming imperceptible at 15 weeks (Figure 2).
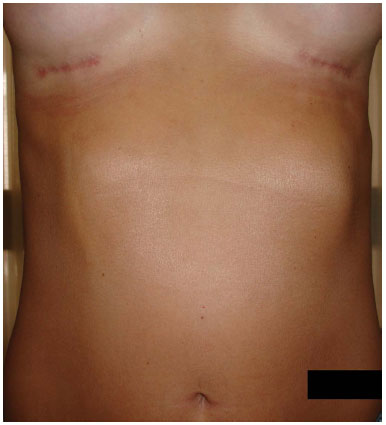
Figure 2. Involution of the fold after 15 weeks.
REFERENCES
1. Spear SL, Parikh PM, Goldstein JA. History of breast implants and the Food and Drug Administration. Clin Plast Surg 2009;36(1):15-21. PMID: 19055957 DOI: http://dx.doi.org/10.1016/j.cps.2008.07.007
2. Sociedade Brasileira de Cirurgia Plástica/Instituto Datafolha. Cirurgia Plástica no Brasil. 2009 [Acesso 10 Set 2015]. Disponível em: http://www2.cirurgiaplastica.org.br/wp-content/uploads/2012/11/pesquisa2009.pdf
3. American Society of Plastic Surgeons Report of the 2010 Plastic Surgery Statistics [Acesso 4 Jul 2012]. Disponível em: http://www.plasticsurgery.org/News-and-Resources/Statistics.html
4. Yang N, Muradali D. The augmented breast: a pictorial review of the abnormal and unusual. AJR Am J Roentgenol. 2011;196(4):W451-60. PMID: 21427311 DOI: http://dx.doi.org/10.2214/AJR.10.4864
5. Moyer HR, Ghazi BH, Losken A. The influence of silicone gel bleed on capsular contracture: a generational study. Plast Reconstr Surg. 2012;130(4):793-800. DOI: http://dx.doi.org/10.1097/PRS.0b013e318262f174
6. Stevens WG, Pacella SJ, Gear AJ, Freeman ME, McWhorter C, Tenenbaum MJ, et al. Clinical experience with a fourth-generation textured silicone gel breast implant: a review of 1012 Mentor MemoryGel breast implants. Aesthet Surg J. 2008;28(6):642-7. DOI: http://dx.doi.org/10.1016/j.asj.2008.09.008
7. Stutman RL, Codner M, Mahoney A, Amei A. Comparison of breast augmentation incisions and common complications. Aesthetic Plast Surg. 2012;36(5):1096-104. DOI: http://dx.doi.org/10.1007/s00266-012-9918-x
8. Stevens WG, Hirsch EM, Tenenbaum MJ, Acevedo M. A prospective study of 708 form-stable silicone gel breast implants. Aesthet Surg J. 2010;30(5):693-701. DOI: http://dx.doi.org/10.1177/1090820X10381880
9. Bozola AR, Bozola AC, Carrazzoni RM. Inclusão de próteses mamárias de silicone - poliuretano. Rev Bras Cir Plast. 2006;21(1):18-22.
10. Khan UD. Incidence of mondor disease in breast augmentation: retrospective study of 2052 breasts using inframammary incision. Plast Reconstr Surg 2008;122(2):88e-9e. PMID: 18626336 DOI: http://dx.doi.org/10.1097/PRS.0b013e31817d6629
1. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
2. Universidade Católica de Pelotas, Pelotas, RS, Brazil
3. Universidade Federal de Pelotas, Pelotas, RS, Brazil
4. Santa Casa de Misericórdia de Porto Alegre, Porto Alegre, RS, Brazil
5. Universidade Federal de São Paulo, São Paulo, SP, Brazil
Institution: Universidade Federal de Pelotas, Pelotas, RS, Brazil.
Corresponding author:
Fernando Passos da Rocha
Praça Piratinino de Almeida, 13, Centro
Pelotas, RS, Brazil Zip Code 96015-290
E-mail: artigosplastica@hotmail.com
Article received: July 7, 2012.
Article accepted: November 15, 2012.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter