

Original Article - Year 2015 - Volume 30 -
Retrospective analysis of treatment approaches used for basal and squamous cell carcinoma in the head and neck
Análise retrospectiva de conduta para carcinoma basocelular e espinocelular em cabeça e pescoço
ABSTRACT
INTRODUCTION: The incidence of skin cancer on the head and neck is increasing worldwide, and basal and squamous cell carcinomas represent the most frequent types. There is no unanimous consensus for all tumor cases, based on the histological type, size, depth, and location of the lesion. The objective is to analyzed the approach used in skin neoplasias in the head and neck, focusing on the treatments performed, recurrence, and follow-up.
METHODS: Sixty-nine patients with basal or squamous cell carcinoma who were treated with surgery, cryotherapy, freezing of lesions in the intraoperative period, or 5% imiquimod were analyzed for 6 weeks. During 36 months of follow-up, the efficacy of the chosen treatment, recurrence, side effects, complications, and esthetic satisfaction of patients were observed. Statistical analysis was performed using the Fisher's exact test.
RESULTS: The most frequent type of reconstruction was primary closure (71%). There were no statistically significant correlations between age, sex, Fitzpatrick classification, location/size of lesion, method of treatment, or recurrence. The main complications resulting from surgery were: a case of a frontal flap necrosis, a partial lesion of the buccinator nerve, and nasal stenosis. There was a 4% tumor recurrence in patients treated with surgery. The cryotherapy and 5% imiquimod treatments resulted in six cases of mild local reactions with a more pronounced recurrence in a patient with superficial basal cell carcinoma (BCC) (not statistically significant).
CONCLUSIONS: Non-superficial BCC and squamous cell carcinomas should be treated with surgery. Superficial BCCs may be treated with cryotherapy and 5% imiquimod with fewer complications and better aesthetic results, but this results in higher tumor recurrence.
Keywords: Skin neoplasias; Basal cell carcinoma; Squamous cell carcinoma; Post-operative complications.
RESUMO
INTRODUÇÃO: O câncer de pele em cabeça e pescoço tem incidência crescente no mundo, sendo o carcinoma basocelular e espinocelular os tipos mais frequentes. Não existe consenso absoluto para todas as situações tumorais conforme tipo histológico, tamanho, profundidade e localização da lesão. O objetivo é analisar a conduta abordada nessas neoplasias de pele em cabeça e pescoço, com ênfase nos tratamentos efetuados, recidivas e seguimento.
MÉTODO: Foram analisados 69 pacientes com carcinoma basocelular ou de células escamosas tratados por cirurgia com congelação da lesão no intraoperatório, crioterapia ou Imiquimod 5% por 6 semanas. Com 36 meses de seguimento, observou-se a eficácia do tratamento escolhido, recidiva, intercorrências, complicações e satisfação estética do paciente. A análise estatística utilizou o teste exato de Fischer.
RESULTADOS: O tipo de reconstrução mais frequente foi o fechamento primário (71%). Não existem associações estatisticamente relevantes relacionando idade, sexo, classificação de Fitzpatrick, local/ tamanho da lesão, métodos de tratamento e recidiva. As principais complicações resultaram das cirurgias: um caso de necrose de retalho frontal, lesão parcial de nervo bucinador, estenose narinária. A recidiva tumoral nos casos operados foi de 4%. A crioterapia e uso do Imiquimod 5% causaram seis casos de reações locais leves com mais recidiva descritiva no tratamento de carcinoma basocelular (CBC) superficial (não estatisticamente relevante).
CONCLUSÕES: Os CBC não superficiais e carcinoma espinocelular devem ser tratados cirurgicamente. Os CBC superficiais podem ser tratados com crioterapia e uso do Imiquimod 5% com menos complicações e melhor resultado estético, mas a recidiva tumoral é maior.
Palavras-chave: Neoplasias cutâneas; Carcinoma basocelular; Carcinoma de células escamosas; Complicações pós-operatórias.
Skin cancer is increasing in incidence all over the world and, according to the National Institute of Cancer (INCA), the official department of the Brazilian Ministry of Health. Skin neoplasias have the highest incidence, with gross national rates of approximately 60 cases/100,000 inhabitants1. Basal cell carcinoma (BCC) is the most common type, accounting for 70-75% of cases. It originates from pluripotent immature epithelial cells that lose the normal capacity to differentiate and keratinize due to various factors, such as the chronic ultraviolet B (UVB) light exposure2-4. Because the head and neck are constantly exposed to sun, these regions are the most common locations for the proliferation of skin neoplasias, especially in tropical countries like Brazil5-7. The second most frequent type of malignant skin cancer is squamous cell carcinoma (SCC), accounting for 15-20% of cases8. It comprises invasive atypical squamous cell proliferation and may result in metastasis. Other etiologic factors contribute to BCC and SCC, such as: burn scars, ulcers, arsenic exposure, ionizing radiation, pigmented xeroderma, HPV infection, and Gorlin and Bazex syndromes8.
Mortality rates due to BCC and SCC are low because these carcinomas rarely metastasize. However, they can be locally aggressive and recurrent8. Early diagnosis is of the utmost importance to prevent large deformities from occurring due to the tumor as well as to enable the use of less aggressive treatment methods.
The suggestive diagnosis is clinical and may be facilitated with the use of dermatoscopy. The histological type classification is performed using incisional or excisional biopsy for lesions greater than 1.0 cm9,10.
Treatments for non-melanoma skin cancer of the face are distinct. The surgical procedure consists of tumor removal with the aid of lesion freezing in the intraoperative period and results in high efficacy with low recurrence. Curettage, electrocoagulation, cryosurgery with liquid nitrogen, 5% imiquimod, and cryosurgery with liquid nitrogen, 5% imiquimod, and topical 5-fluorouracil may be options for superficial BCCs smaller than 1.0 cm8. For patients without clinical conditions with extensive tumors, radiotherapy may be an alternative. In SCC with metastasis and aggressive tumors with metastasis to the lymph nodes, a lymphadenectomy should be performed and complemented with radiotherapy. If radiotherapy use is impossible, regional intra-arterial chemotherapy, which allows high concentrations of chemotherapeutic agents in the tumor area, minimizing its collateral effects, can be an option8-10.
Although there are specific approaches for skin cancer treatment, there is no unanimous consensus for all tumor cases, with respect to the histological type, size, depth, and location of the lesion. Among plastic surgeons, the most frequent approach to treat skin tumors is surgery; however, other less invasive approaches have been shown to have good results using dermatological procedures, especially for superficial BCC cases. The rates of compromised margins in surgery vary from 4% to 41% in the international literature11-17. In medical practice, in addition to being guided by the oncologic approaches, we must also consider the acceptance of surgical treatment by the patient, as well as recovery type, financial cost, location of the scar, histological type of lesion, and life expectancy. This work aims to analyze the approaches used for BCCs and SCCs in the head and neck, while taking into consideration this diversity of factors and always prioritizing full tumor cure with the best aesthetic results possible.
OBJECTIVE
This study reports on the approaches used in 69 cases of basal and squamous cell carcinomas in the head and neck, with an emphasis on the treatments performed, recurrences, and follow-up.
METHODS
In this work, 69 patients (51 females and 18 males) with skin tumors were selected, and patients were an average age of 59.4 years old. All patients had lesions that were clinically suggestive of SCC or BCC based on the topography of the head and neck, and this was confirmed by biopsy. Patients were randomly selected, according to the following exclusion criteria: pregnant women, patients younger than 45 years old or over 91 years old, immunosuppressed individuals, patients undergoing any treatment for malignant tumors, patients using chemotherapy, and patients with uncontrolled systemic diseases, including systemic arterial hypertension, type I or II diabetes, and hypo/hyperthyroidism.
This retrospective study used data from 69 patient records from a randomized sample. The patients were treated in 2008 and 2009. Patients who were not excluded using the above cited criteria were accepted to participate in the study after filling out and full understanding of the informed consent form, which made it clear that their identities would not be revealed. Additionally, the photographs to be used were partially altered to minimize their identification.
The approach used for tumor lesions is represented in the flowchart in Figure 1.

Figure 1. Flowchart of the adopted treatment approach for basal and squamous cell carcinomas of the head and neck. SCC: Squamous Cell Carcinoma.
The surgical approaches for non-superficial SCCs and BCCs followed the oncologic guidelines, with full excision of the tumor lesion followed by the analysis of the lesion after freezing. Tumors ranged in size from 2 to 21 mm in diameter and were operated on with 3-mm (BCC) to 7-mm (ulcerated SCC) surgical margins. The margins were described by the pathologist as "free", "compromised", or "exiguous" (when the resection limit was close to the neoplastic cell location). The "compromised" and "exiguous" margins were expanded and analyzed again by freezing until they were "free of neoplasia". Then, the resulting defect was closed using the techniques described in Figure 2.
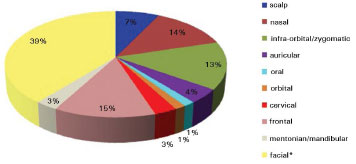
Figure 2. Topographic regions of basal and squamous cell carcinomas (*facial region corresponds to the area situated between the infraorbital/zygomatic and mentonian/mandibular regions).
The 5% imiquimod treatment for superficial BCC was explained during medical appointments by the author and was recommended to be performed at home. The treatment was conducted three times a week (Monday, Wednesday, and Friday) with the application of a thin layer of the product over the affected area before bedtime by rubbing until the product was fully absorbed. The site was not occluded and remained active for 6 to 10 hours. The product was removed the following morning, and the area was washed with water and neutral soap. The next appointment was 15 days later. This approach was repeated until a clinical cure was observed, and treatment was suspended if any complications/side effects occurred or if it was unsuccessful after 6 weeks.
Cryotherapy on superficial BCC was performed using the liquid nitrogen spray atomization technique over the lesion with a specific instrument (Cry-Ac®). Application was performed intermittently until the entire superficial tumor lesion was frozen for 60 seconds.
Treatment selection for superficial BCC cases was made along with the patient, after explaining the eventual benefits, risks, and side effects of each approach.
In the remaining histological types, surgery was performed based on location, size of the lesion, and resulting defect.
All patients were photographed with a Nikon D60 camera using AF-S Micro Nikkor 60 mm 1: 2.8 g ED and AF-S Nikkor 55-200 mm 1:4-5.6 g ED lenses.
Follow-up was performed in medical appointments for 36 months, and thief a patient was unable to return for an appointment, the follow-up was performed by phone. The patients that were not able to attend medical appointments were asked to allow alternative reliable plastic surgeons or dermatologists to evaluate them in order to provide information on cure and tumor recurrence. The data from the records analyzed were: age, sex, occupation, sun-protection habits (use of sunscreens, sunglasses, and hats), Fitzpatrick classification, location and size of the lesion, number of lesions, biopsy results, definitive result of pathological anatomy, treatment methods, recurrence, and satisfaction with the aesthetic result.
Histologically, BCCs were divided into nodular, micronodular, sclerodermiform, ulcerated, adenoid, superficial, and pigmented subtypes. SCCs were evaluated in a progressive order of malignancy, according to the Broder's classification: Grade I, 0 to 25% undifferentiated cells; Grade II, 25 to 50% undifferentiated cells; Grade III, 50 to 75% undifferentiated cells; and Grade IV, 75 to 100% undifferentiated cells.
The patient's satisfaction with the aesthetic result was analyzed in a subjective manner, with the patient responding to the following question given to them by the receptionist or sent by email/letter: "Do you consider your aesthetic result from treatment to be: poor, intermediate, good, or great?" The responses were marked by circling one of the four options, and they were sent back to the receptionist.
The results were submitted for statistical analysis using the Fisher's exact test, with a significance level of 5%.
RESULTS
There was no statistically significant correlation between occupation and sun-protection habits. All patients with histological samples submitted from surgery had histological confirmation by biopsy with definitive pathological anatomy (paraffin).
The topographic regions of the BCC and SCC are represented in Figure 2. Of the 69 patients, five had multiple superficial BCC lesions at the first appointment; the multiple lesions were not included in the study since they were being clinically followed by the patients' dermatologists, who conducted a nonsurgical treatment approach. All patients were treated surgically, except for 18 patients with superficial BCC that opted for clinical treatments with a long-term follow-up. Seven of those patients were treated with 5% Imiquimod, and the 11 remaining patients were treated with cryotherapy. Figure 3 shows the surgical techniques used in the 51 cases submitted to surgery.

Figure 3. Distribution (in absolute number) of the surgical procedures, according to the technical procedures used. *Composite grafts of skin and auricular cartilage; **Indian flap (frontal) in two surgical steps; ***Indian flap (frontal) in three surgical steps; ****Fusiform resection followed by simple direct closure; ***** Lateral rotation of graft in the dissection plane of a facelift.
The patients in this had a high incidence of BCC (97%) compared to that of SCC (3%), and there was no statistically higher incidence with respect to sex or age. For ulcerated BCC, we found an important correlation with age: 11 patients (92%) were between 73 and 91 years old. Table 1 shows the absolute distribution of basal and squamous cell carcinoma histological types in the study, recurrence after treatment, and side effects/complications. The two cases of SCC were Grade II by Broder's classification. The worst surgical complications observed were a buccinator nerve lesion with partial improvement 1 year after surgery and necrosis of the frontal flap used to reconstruct the nasal tip, which is expected to be reconstructed after the patient quits smoking. Nasal stenosis after surgery with composite grafts of skin and cartilage and unevenness with discrete hyperchromia in the nasal dorsum skin graft were also observed (Figure 4).
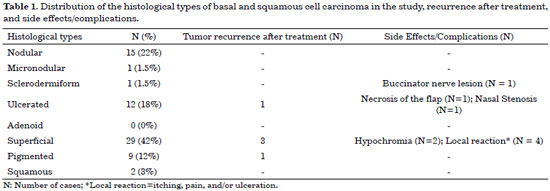

Figure 4. Surgical complications and undesirable results. In A, B, and C, data from pre-, intra-, and post-operative periods of facial tumor cases are shown, respectively. In D, a total skin graft in dorsum with hyperchromia and unevenness in the subjacent tissues is shown. In E, early necrosis in the first stage of frontal flap reconstruction (smoking patient) is shown. In F, a composite graft in left nostril domed in the direction of the introitus, resulting in moderate nasal stenosis, is shown.
Based on the Fitzpatrick classification, groups 3 and 4 were the most detected, at 58% and 25% of all cases, respectively.
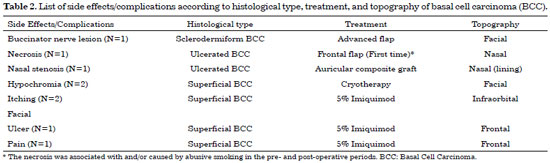
Figure 5 shows tumor recurrence according to the histological type and treatment and topography of the lesion. Table 2 lists side effects/with respect to histological type, treatment, and topography of the skin tumor.

Figure 5. List of tumor recurrences according to histological type, treatment, and topography. BCC: Basal Cell Carcinoma.
After the first tumor resection, histopathological analysis of samples during surgery showed 74% "free", 14% "compromised" and 12% "exiguous" margins. Cases with "compromised" and "exiguous" margins were expanded again during that same surgery, with only three cases requiring extension for a third time to obtain a "free" margin. In final pathological analysis of paraffin-embedded tissue, two cases (ulcerated BCC and pigmented BCC) actually had "exiguous" margins.
The Abbé flap is shown in Figure 6 with details of surgical markings, the intraoperative period, and unsectioned pedicle. Figure 7 illustrates cases in the glabellar and nasolabial flaps, and Figure 8 shows the preparation of the Rintala flap. The case in which surgery was performed in the posterior auricular flap (Masson) is shown in Figure 9, and that in which surgery was performed in the bilobed flap on the nose is shown in Figure 10. The most complex and longest surgery was the reconstruction of the nasal defect performed with frontal flap septal cartilage to sustain the right nasal wing (Figures 11 and 12).

Figure 6. Abbé flap. In A, surgical markings are shown. In B, the flap rotation when trying to align the cutaneous-mucosal line is shown. In C, the aspect of the non-sectioned pedicle is shown. In D, the final result is shown.

Figure 7. In A and B, the glabellar flap is shown. In C and D, the nasolabial flap is shown. We observed that both resulted in a greater volume than required in the receiving area, resulting in little aesthetic refinement.

Figure 8. Rintala flap. In A, surgical markings are shown. In B, we observed a great detachment of the flap to allow its caudal advance in the direction of the defect. In C and D, the aspect of the defect and its reconstruction are shown, respectively.

Figure 9. The posterior auricular flap isolated in the "hinge". In A, the defect caused by the tumor resection is shown. In B, the path that the skin flap will go through is shown. In C, the island of posterior auricular skin that will be rotated for the reconstruction of the defect is shown. In D, the final aspect of the flap is shown.

Figure 10. Bilobed flap. In A and B, pre- and postoperative flaps are shown, respectively. In C, intraoperative markings are shown. In D, the rotations of the flaps are shown.

Figure 11. Frontal flap. In A, the inclusion of the septal graft to sustain the right nasal wing is shown. In B, the marking of the subunit of the nasal tip transferred to an ipsilateral aluminum model for blood suppression, as chosen by Doppler, is shown. In C, the rotation of the flap, observing no excessive tension or torsion of the pedicle, is shown. In D, the marking before the pedicle section in order to not distort the supercilium is shown. In E, the supercilium in the normal position and a muscular/fat composition in the pedicle are observed. In F, the third stage of reconstruction with flap thinning is shown.

Figure 12. Details of the frontal flap. In A, the marking is shown of the aesthetic subunits to be preserved during reconstruction with special attention given to the "fragile triangle" in this case. In B, the use of Doppler to select the best option of pedicle is shown. In C, we observed the skin graft in the posterior surface of the flap. In D, E, and F, the aspects of the defect, intermediate, and final results, respectively, are shown.
Patients thought that the aesthetic results after treatment were poor (6%), intermediate (38%), good (48%), or great (8%).
We compared the proportions of recurrence in groups of patients who either underwent surgery or not. Fisher's exact test was used, which resulted in a p value of 0.107. At a significance level of 5%, the null hypothesis that the populations were identical was not rejected. With respect to tumor recurrence based on treatment (having surgery or not), descriptively, there was a difference, with those not having surgery (3/18) having a 16.7% recurrence and those that underwent surgery (2/51) having a 3.9% recurrence. However, statistical tests did not detect a difference in populations.
DISCUSSION
Skin neoplasia is very frequent on the face and neck, especially in patients over 60 years old1,5-7,18. Non-melanoma skin cancer has multiple risk factors, and, in our case series, being over 60 years old and having a history of sun exposure without adequate sun protection were the most notable. The elderly immunological system experiences difficulties in DNA regeneration, which facilitates the development of the BCC and SCC types of skin cancer8.
Individual susceptibility also influences the appearance of tumors. Our study agrees with international statistical data, and phototypes I to III in the Fitzpatrick classification were the most affected. In our study, we did not have cases with a great risk of tumor development such as that occurring in individuals with basal-cell nevus syndrome, albinism, or pigmented xeroderma8.
In this study, skin cancer occurrence was more frequent in females (74%). However, literature varies according to the area where the study was performed and the sample, with a possible higher incidence in males found in some publications3,4. Brazil, our study found some common characteristics of the most common histological type facial BCC, with the nodular/ulcerated type being the most frequent subtype in the INCA study as well as our study (excluding the superficial BCC cases).
The literature shows that 40% to 71.2% cases of BCC and SCC tumors are located in the head and neck region. Our study showed the most affected locations were the facial (39%), frontal (15%), and nasal (14%) regions. This differs from the literature, which states that the nose is the most frequently affected region of the face (30-35% of cases)5,6.
Therapy with 5% Imiquimod has been used successfully in Brazil for the treatment of several dermatological conditions, including basal cell carcinoma, Bowen disease, contagious mollusk and viral warts19,20. Imiquimod is registered in Brazil for the treatment of external warts present in the genital and perianal regions (condyloma acuminata), in patients 12 years old or older, and also is also approved for the treatment of keratosis actinic19,20. The literature described curing of superficial BCC with the use of Imiquimod varying from 78.4% to 90%21-30, with the quick response with the treatment being a predictor of long term efficacy. Imiquimod strengthens interferon production, which has an antiviral, antiproliferative and antiangiogenic effect. It also stimulates the Langerhans cells, the main cells with epidermis antigens, to migrate to the lymph nodes and activate the production of HPV-specific T cells23,26. Imiquimod is also a biological response modifier, mimicking what occurs in the normal immune response when HPV is recognized by the immune system28.
In our study, the use of 5% Imiquimod was well justified, but some elderly patients had a bit of difficulty understanding it. This may have affected the treatment performed with this drug at home. Applying the product and going to bed right afterwards was a common error, as the product could be lost on the pillow or even spread to unwanted areas of the face. Care was required to not apply the product near the eyes, in order to avoid unwanted ocular reactions. When crusts in the lesions appeared during the drug treatment period, we suggested use of a gentamycin cream.
There is no consensus in the literature on the regimen of 5% Imiquimod use, which may vary from three applications per week to daily use. However, erythema and itching are more common with a greater number of applications24-27. In our study, one patient presented with ulceration, which has also been described in literature23-30. We chose to suspend drug use for some days when side effects occurred, and, after improvement, we restarted treatment. We opted for a 6 week-long treatment regimen, but this can be extended according to needs20,25,31,32. The absence of tumor recurrence after 5% Imiquimod treatment resulted in satisfaction from patients, even with some cases side effects during treatment.
Cryotherapy has increased in interest after the development of new instruments, better knowledge about technique and better defined recommendations. It can be used on small, medium, and large lesions, both as the treatment of choice or for palliation of tumors that cannot undergo surgery33-35. Basic knowledge in cryosurgery, selection by patients, and knowledge about the immediate and late post-operative period are of utmost importance to successful use of this method. The action mechanism of freezing in the lesion tissue is achieved through intraand extracellular osmotic imbalance. When temperatures fall in the cell, ice microcrystal formation will occur. When tissues thaw, the microcrystals, since they have a great amount of free energy, undergo re-crystallization, and form macrocrystals, which rupture cell walls, from the inside of the cell outwards36.
Cryotherapy progressed rapidly, developing several techniques and modifying others, to obtain very satisfactory final results. Application techniques may be diverse and include using a stylus with cotton tip (Deepstik), atomization "spray", atomization using a neoprene or plastic cone and the solid contact technique (a closed system connected to the application instrument)34. In our study, we opted to use the atomization "spray" technique due to the great experience of the author in conducting it. The need to monitor the freezing process was a relevant criticism about the cryotherapy technique.
The thermoregulation needle method uses hidroidrias needles that have a temperature sensor in the tip. It is a safe and effective method to evaluate depth and laterality of the freezing face, and it allows for measurement of the temperature reached. For malignant tumors, a temperature between -25ºC and -60ºC is desirable. Another way to measure freezing is the electrical resistance/impedance method, in which the resistance that the mass of the frozen tissue offers to the passage of low voltage electric flow is measured with a simple electrode36. Similar to what happens in services use cryotherapy, our study did not monitor freezing, which could explain the high incidence of recurrence of superficial BCCs (28%) and two cases of hypochromia. This is therefore not satisfactory oncologic treatment, and only its low cost associated was considered by the patient.
This study aimed to demonstrate some treatment options in private skin oncology clinical practice. The selection of patient treatment regimens should be a bilateral agreement between doctor and patient, considering the diverse particularities of each case and focusing on tumor treatment and good aesthetic results. The structural differences of different tissues in the various anatomical regions, based on cartilage or bone, vascularization, and keratinized surfaces, led to the best results after cryosurgery and 5% Imiquimod treatment. Conjunctive tissue (bone, cartilage, and fibroblasts) is much more resistant to temperature and Imiquimod than other structures in this tissue, such as the dermis and epidermis, allowing destruction of tumor structures located in them without injuring the underlying tissue and providing a cosmetically favorable scar37-39. In some situations, not performing surgery may be a good alternative, such as for patients with lesions located in the ear and nose, elderly patients or those with multiple lesions, those who have a pacemaker or use anticoagulants, anxious individuals, and patients that panic during surgery39. Furthermore, larger superficial lesions may be treated by follow-up procedures, avoiding hospitalization and more extensive surgeries. These situations should be discussed openly with patients, weighing the benefits and risks of greater recurrence when compared to surgery.
The two cases of recurrence observed in the definitive anatomopathological exam with "exiguous" margins are under constant clinical follow-up, with the patients consent, since through dermatoscopy, there have been no signs of recurrence. This approach agrees with recommendations from the literature, with the possibility of recurrence being more frequent in lateral and non-deep margins. Most studies showed that the margins most affected were lateral (62%), followed by deep margins (34%). Our study showed that only cases with lateral margins had compromised margins. Furthermore, studies have revealed that only one-third of patients with tumors with positive margins in the primary excision presented with residual disease when undergoing an additional surgery40,41. Even with this information, we emphasized to the patient the need of performing follow-up with the plastic surgeon or dermatologist. For elderly patients, recurrence in 5-10 years may occur with adverse clinical conditions that hinder a surgical approach.
Some studies found recurrence rates of up to 26% for resected skin tumors with compromised margins and up to 14% for fully resected lesions in 5 years11. For this reason, there is no consensus on which medical approach should be used in patients with a higher surgical risk and low life expectancy, such that conservative treatment constitutes a possible alternative. In our study, recurrence upon incomplete resection of the tumor was 4%, which was a bit lower than that found in the literature, which varies from 4.7% to 18.2%12-17. Recurrence in our patients occurred in the facial and auricular regions in a statistically insignificant manner, but our data were not in agreement with the literature, which notes that the nasal region is the site of the highest rate of tumor recurrence, varying from 9% to 26%11-17. The aesthetic importance of the nose could be a possible reason for this high rate of reccurence42-44.
The surgical resections used initial resection margins according to the data in the literature, varying from 3 to 5 mm for BCC and from 5 to 7 mm for SCC, followed by immediate extension as needed8-10. The surgical resection technique was easier in histological subtypes with well-defined margins, such as those that occur in nodular and ulcerated BCC11. Studies showed that the superficial and sclerodermiform types recurred more often, due to the difficulties in delimiting the margins to be resected12-14. The eyelids, nose, and ears were also more likely to have compromised margins due to the aesthetic importance of these areas.
Surgical reconstruction with primary closure was most commonly used on our patients (71%), followed by the use of flaps (36%) and skin grafts (3%). Most studies in the literature showed the same order of preference for reconstruction, but with percentages varying from 48-87.4%, 6.5-22.1%, and 2.8-18.5%, respectively2-5. This follows logically since the surgeon must always try to reconstruct the defect in the easiest and least aggressive way, which is by using primary closure. The greatest exception is nose reconstruction, in which the aesthetic subunits described by Burget and Menick should be preserved45,46. The transversal closure near the dorsum near the nasal root yields good results, but in other regions, this may elevate the nasal tip and/or cause asymmetry. Each nasal subunit should be reconstructed in full, and, in cases where the multiple subunits are affected, all subunits should be reconstructed at the same time47-55. In one patient, in which we used the Rintala flap for nasal tip reconstruction, the scar was aesthetically acceptable, but the unwanted effect of increasing the columella-labial angle was observed.
The three cases of nasal defect in the lateral wall reconstructed with a bilobed flap resulted in a great aesthetic result, while not altering the height of the nose margin. In the two cases of frontal flap reconstruction, we observed great ease in the reconstruction refinement in three times, as it permitted making the flap "thinner" and improved the aesthetics. In the case of necrosis, which was encountered twice in frontal flap reconstruction, we believe the patient's intense smoking was the cause. This was not revealed in the anamnesis as the main cause of this complication, since we used Doppler to confirm the presence of the supratrochlear artery, and no lesion of this artery occurred during surgery. We believe that performing a nicotine urine test will help identify patients who smoke, but this exam is not performed in the majority of laboratories in Brazil. The choice between performing frontal flap reconstruction in two or three steps should take the anxiety of patient and the cost of the procedure into consideration. However, reconstruction in three steps provides a notably better aesthetic result. We considered reconstruction of the nasal lining using composite grafts of cartilage and auricular skin very difficult because the access path is small for the fixation of the cephalic portion of the graft. This technical difficulty has probably resulted in a dead space that caused local fibrosis and, subsequently, nasal stenosis. As an option for nasal lining reconstruction, the literature cites a local advance in V-Y or the frontal flap54.
In addition to frontal flap necrosis and nasal stenosis, an additional surgical complication was the buccinator nerve lesion that occurred during a SCC resection in the facial region. The surgery occurred without apparent complications, with detachment of the flap in the rhytidoplasty plane without visualizing the affected nerve. Because symptomatic improvement occurred after a year, we concluded that it was a partial lesion.
The overall aesthetic results of the procedures in which flaps were used were satisfactory. However, the only case of skin graft reconstruction resulted in a local depression, or unevenness in relation to neighboring tissues, and change in skin color, leaving a stigma of the surgery. For these reasons, whenever possible, we avoid the use of grafts in the reconstruction of face and neck defects. The posterior auricular flap was a good alternative to reconstruct the auricular defect. The patient with superior labial reconstruction with the Abbé flap progressed well, but the cutaneous- mucosal line on the side that was operated on was still not symmetrical, such that filling it with fat or hyaluronic acid might be a less traumatic option for optimizing the result of this surgery in the future.
It was surprising how the possible aesthetic results of treatment influenced patients' decisions. Our study showed that even without the patient having prior experience using 5% Imiquimod or cryotherapy, many were more concerned about the aesthetic results and unwanted effects of surgery than with the treatment cost and cure. This means that, an alternative treatment using "cream" or "cryotherapy" was more attractive than surgical intervention, which had a better chance of cure. This should be taken into consideration by doctors that treat skin neoplasias in order to avoid recommending a less adequate treatment due to the patient opinion. Each case needs to be evaluated by the doctor and should include his professional opinion, aiming at the better option for cure and aesthetic outcome56,57.
We considered the fact that the participants were not previously treated with 5% Imiquimod or cryotherapy a limitation of this study. Thus, these patients did not have real access to the possible collateral reactions or effects of treatment, and it is more difficult to propose a surgical technique in a situation where a dermatological procedure seems favorable. We believe a future study about this same subject that aims to elucidate the preference of treatment types with a greater number of patients that have already been treated using the three approaches under examination in the present study would be of great value. The great advantage of topical treatment of superficial BCC is that it is an option for patients for whom surgery is not recommended or those with multiple lesions. These treatments also represent a low cost alternative with tolerable side effects. However, we should emphasize that monitoring of patients not treated with surgery should be maintained long-term, especially for those with lesions that do not completely respond to treatment. The possible reason may be due to the presence of mixed histology lesions, even if the biopsy showed that is the lesion was only a superficial BCC.
The average time of patient follow-up in this study was 36 months, which was based on the average time of recurrence of 35 months in other scientific studies. Nevertheless, we recommend that our oncologic patients be followed for at least 60 months since late recurrence may occur. The National Institute of Cancer (INCA) recommended extending surgical margins only when more than one affected positive margin or deep margin was detected in 200512. This study reinforces such an approach, along with the support of other scientific studies that defend the possibility of clinical follow-up for BCC patients. For SCC patients, we do not recommend an observational approach.
CONCLUSIONS
The analysis in this study of treatment approaches for basal and squamous cell carcinomas on the head and neck suggests that oncologic treatment may be individualized according to histological type/size/depth/ location of the lesion, patient financial adequacy, and life expectancy. Non-superficial BCC and SCC should be treated with surgery. Superficial BCC may be treated with surgery or non-invasive procedures, such as cryotherapy and 5% Imiquimod. The patient should be aware that superficial BCC recurrence is more likely with a conservative approach although it can offer better aesthetic results and fewer complications compared to surgical procedures.
REFERENCES
1. Brasil. Ministério da Saúde. Instituto Nacional do Câncer. Estimativa 2008: Incidência de câncer no Brasil [Acesso 14 Nov 2015]. Disponível em: http://www.inca.gov.br/conteudo_view.asp?id=1793
2. Telfer NR, Colver GB, Morton CA; British Association of Dermatologists. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159(1):35-48. PMID: 18593385 DOI: http://dx.doi.org/10.1111/j.1365-2133.2008.08666.x
3. Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353(21):2262-9. PMID: 16306523 DOI: http://dx.doi.org/10.1056/NEJMra044151
4. Thissen MR, Neumann MH, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135(10):1177-83. DOI: http://dx.doi.org/10.1001/archderm.135.10.1177
5. Santos ABO, Loureiro V, Araújo Filho VJF, Ferraz AR. Estudo epidemiológico de 230 casos de carcinoma basocelular agressivos em cabeça e pescoço. Rev Bras Cir Cabeça Pescoço. 2007;36(4):230-3.
6. Kligerman J. Estimativas sobre a incidência e mortalidade por câncer no Brasil. Rev Bras Cancerol. 2002;48(2):175-9.
7. Nasser N. Epidemiologia dos carcinomas basocelulares em Blumenau, SC, Brasil, de 1980 a 1999. An Bras Dermatol. 2005;80(4):363-8. DOI: http://dx.doi.org/10.1590/S0365-05962005000400006
8. Sampaio SAP, Castro RM, Rivitti EA. Dermatologia Básica. São Paulo: Artes Médicas; 2007.
9. Dini GM, Ferreira LM. Correção das deformidades nasais com o uso de cartilagem costal. In: Ferreira LM, org. Guia de medicina ambulatorial e hospitalar da UNIFESP: Cirurgia Plástica Estética. 1a ed. Barueri: Manole; 2007. p.355-60.
10. Neves RI, Brechtbühl ER, Almeida OM. Tumores malignos da pele. Rev Bras Cir Plást. 2005;77:799-801.
11. Quintas RCS. Coutinho ALF. Fatores de risco para o comproetimento de margens cirúrgicas nas ressecções de carcinomas basocelular. Rev Bras Cir Plást. 2008;23(2):116-19.
12. Gregorio TCR, Sbalchiero JC, Leal, PRA. Acompanhamento a longo prazo de carcinomas basocelulares com margens comprometidas. Rev Soc Bras Cir Plást. 2005;20(1):8-11.
13. Berlin J, Katz KH, Helm KF, Maloney ME. The significance of tumor persistence after incomplete excision of basal cell carcinoma. J Am Acad Dermatol. 2002;46(4):549-53. PMID: 11907506 DOI: http://dx.doi.org/10.1067/mjd.2002.117733
14. Holmkvist KA, Rogers GS, Dahl PR. Incidence of residual basal cell carcinoma in patients who appear tumor free after biopsy. J Am Acad Dermatol. 1999;41(4):600-5. PMID: 10495384
15. Pascal RR, Hobby LW, Lattes R, Crikelair GF. Prognosis of incompletely excised versus completely excised basal cell carcinoma. Plast Reconstr Surg. 1968;41(4):328-32. PMID: 5647401
16. Nagore E, Grau C, Molinero J, Fortea JM. Positive margins in basal cell carcinoma: relationship to clinical features and recurrence risk. A retrospective study of 248 patients. J Eur Acad Dermatol Venereol. 2003;17(2):167-70. DOI: http://dx.doi.org/10.1046/j.1468-3083.2003.00535.x
17. Thomas DJ, King AR, Peat BG. Excision margins for nonmelanotic skin cancer. Plast Reconstr Surg. 2003;112(1):57-63. PMID: 12832877 DOI: http://dx.doi.org/10.1097/01.PRS.0000067479.77859.31
18. Bandeira AM, Bandeira V, Silva JF, Mazza E. Carcinomas basocelulares: estudo clínico e anatomopatológico de 704 tumores. An Bras Dermatol. 2003;78(1):23-34. DOI: http://dx.doi.org/10.1590/S0365-05962003000100003
19. Alessi SS, Sanches JA, Oliveira WR, Messina MC, Pimentel ER, Festa Neto C. Treatment of cutaneous tumors with topical 5% imiquimod cream. Clinics (Sao Paulo). 2009;64(10):961-6. DOI: http://dx.doi.org/10.1590/S1807-59322009001000005
20. Festa Neto C. Tratamento tópico do carcinoma basocelular superficial e nodular pelo imiquimode creme a 5%: observação de 10 casos. An Bras Dermatol. 2002;77(6):693-8. DOI: http://dx.doi.org/10.1590/S0365-05962002000600006
21. Ozolins M, Williams HC, Armstrong SJ, Bath-Hextall FJ. The SINS trial: a randomised controlled trial of excisional surgery versus imiquimod 5% cream for nodular and superficial basal cell carcinoma. Trials. 2010;11:42. DOI: http://dx.doi.org/10.1186/1745-6215-11-42
22. Rigel DS, Torres AM, Ely H. Imiquimod 5% cream following curettage without electrodesiccation for basal cell carcinoma: preliminary report. J Drugs Dermatol. 2008;7(1 Suppl 1):s15-6.
23. Shumack S, Robinson J, Kossard S, Golitz L, Greenway H, Schroeter A, et al. Efficacy of topical 5% imiquimod cream for the treatment of nodular basal cell carcinoma: comparison of dosing regimens. Arch Dermatol. 2002;138(9):1165-71. PMID: 12224977 DOI: http://dx.doi.org/10.1001/archderm.138.9.1165
24. Gollnick H, Barona CG, Frank RG, Ruzicka T, Megahed M, Maus J, et al. Recurrence rate of superficial basal cell carcinoma following treatment with imiquimod 5% cream: conclusion of a 5-year long-term follow-up study in Europe. Eur J Dermatol. 2008;18(6):677-82.
25. Marks R, Gebauer K, Shumack S, Amies M, Bryden J, Fox TL, et al. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: results of a multicenter 6-week dose-response trial. J Am Acad Dermatol. 2001;44(5):807-13. DOI: http://dx.doi.org/10.1067/mjd.2001.113689
26. Raasch B. Management of superficial basal cell carcinoma: focus on imiquimod. Clin Cosmet Investig Dermatol. 2009;2:65-75. DOI: http://dx.doi.org/10.2147/CCID.S3507
27. Schön MP, Schön M. Imiquimod: mode of action. Br J Dermatol. 2007;157 Suppl 2:8-13. PMID: 18067624
28. Quirk C, Gebauer K, Owens M, Stampone P. Two-year interim results from a 5-year study evaluating clinical recurrence of superficial basal cell carcinoma after treatment with imiquimod 5% cream daily for 6 weeks. Australas J Dermatol. 2006;47(4):258-65. PMID: 17034468 DOI: http://dx.doi.org/10.1111/j.1440-0960.2006.00313.x
29. Vidal D, Matías-Guiu X, Alomar A. Fifty-five basal cell carcinomas treated with topical imiquimod: outcome at 5-year follow-up. Arch Dermatol. 2007;143(2):266-8. PMID: 17310012 DOI: http://dx.doi.org/10.1001/archderm.143.2.266
30. Wu JK, Oh C, Strutton G, Siller G. An open-label, pilot study examining the efficacy of curettage followed by imiquimod 5% cream for the treatment of primary nodular basal cell carcinoma. Australas J Dermatol. 2006;47(1):46-8. PMID: 16405483 DOI: http://dx.doi.org/10.1111/j.1440-0960.2006.00222.x
31. Sterry W, Ruzicka T, Herrera E, Takwale A, Bichel J, Andres K, et al. Imiquimod 5% cream for the treatment of superficial and nodular basal cell carcinoma: randomized studies comparing low-frequency dosing with and without occlusion. Br J Dermatol. 2002;147(6):1227-36. PMID: 12452875 DOI: http://dx.doi.org/10.1046/j.1365-2133.2002.05069.x
32. Schulze HJ, Cribier B, Requena L, Reifenberger J, Ferrándiz C, Garcia Diez A, et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from a randomized vehicle-controlled phase III study in Europe. Br J Dermatol. 2005;152(5):939-47. PMID: 15888150 DOI: http://dx.doi.org/10.1111/j.1365-2133.2005.06486.x
33. Thissen MR, Nieman FH, Ideler AH, Berretty PJ, Neumann HA. Cosmetic results of cryosurgery versus surgical excision for primary uncomplicated basal cell carcinomas of the head and neck. Dermatol Surg. 2000;26(8):759-64. DOI: http://dx.doi.org/10.1046/j.1524-4725.2000.ds00064.x
34. Peikert JM. Prospective trial of curettage and cryosurgery in the management of non-facial, superficial, and minimally invasive basal and squamous cell carcinoma. Int J Dermatol. 2011;50(9):1135-8. DOI: http://dx.doi.org/10.1111/j.1365-4632.2011.04969.x
35. Antunes AA, Antunes PA, Silva PV. Papel da criocirurgia no tratamento das neoplasias cutâneas do seguimento cabeça e pescoço: análise de 1900 casos. Rev Col Bras Cir. 2006;33(2):112-5. DOI: http://dx.doi.org/10.1590/S0100-69912006000200011
36. Torre D. Cryosurgery of basal cell carcinoma. J Am Acad Dermatol. 1986;15(5 Pt 1):917-29.
37. Raasch BA, Buettner PG, Garbe C. Basal cell carcinoma: histological classification and body-site distribution. Br J Dermatol. 2006;155(2):401-7. PMID: 16882181 DOI: http://dx.doi.org/10.1111/j.1365-2133.2006.07234.x
38. Nagore E, Grau C, Molinero J, Fortea JM. Positive margins in basal cell carcinoma: relationship to clinical features and recurrence risk. A retrospective study of 248 patients. J Eur Acad Dermatol Venereol. 2003;17(2):167-70. DOI: http://dx.doi.org/10.1046/j.1468-3083.2003.00535.x
39. Raasch B, Woolley T. Management of primary superficial basal cell carcinoma. Aust Fam Physician. 2006;35(6):455-8. PMID: 16751865
40. Zide MF, Adnot J. Delayed treatment of patients with multiple facial skin cancer defects: the effect of setting. J Oral Maxillofac Surg. 2008;66(7):1545-50. PMID: 18571050 DOI: http://dx.doi.org/10.1016/j.joms.2007.11.022
41. Farhi D, Dupin N, Palangié A, Carlotti A, Avril MF. Incomplete excision of basal cell carcinoma: rate and associated factors among 362 consecutive cases. Dermatol Surg. 2007;33(10):1207-14. DOI: http://dx.doi.org/10.1097/00042728-200710000-00008
42. Turan A, Kul Z, Türkaslan T, Ozyiğit T, Ozsoy Z. Reconstruction of lower half defects of the nose with the lateral nasal artery pedicle nasolabial island flap. Plast Reconstr Surg. 2007;119(6):1767-72. PMID: 17440352 DOI: http://dx.doi.org/10.1097/01.prs.0000259088.47033.aa
43. Wesley NO, Yu SS, Grekin RC, Neuhaus IM. Primary linear closure for large defects of the nasal supratip. Dermatol Surg. 2008;34(3):380-4.
44. Veríssimo P, Barbosa MVJ. Tratamento cirúrgico de tumores de pele nasal em idosos. Rev Bras Cir Plást. 2009;24(2):219-33.
45. Burget GC, Menick FJ. The subunit principle in nasal reconstruction. Plast Reconstr Surg. 1985;76(2):239-47. PMID: 4023097 DOI: http://dx.doi.org/10.1097/00006534-198508000-00010
46. Menick FJ. The evolution of lining in nasal reconstruction. Clin Plast Surg. 2009;36(3):421-41. PMID: 19505612 DOI: http://dx.doi.org/10.1016/j.cps.2009.02.014
47. Oliveira Júnior FC, Figueiredo JCA, Piva AM. Técnicas de reconstrução cutânea aplicadas às subunidades estéticas nasais. Rev Bras Cir Craniomaxilofac. 2009;12(3):105-8.
48. Lima BS, Abdalla SC, Accioli ZV, Accioli VJJ, Vieira V, Bins-Ely J, D'éça RN. Reconstrução nasal com retalho frontal: nossa experiência. Arq Catarin Med. 2007;36(supl.1):103-5.
49. Rocha FP, Fagundes DJ, Almeida MWR, Costa TV, Pires JA. Retalho nasolabial versátil em cirurgia de reconstrução de nariz. Rev AMRIGS. 2010;54(2):190-3.
50. Laitano FF, Teixeira LF, Siqueira EJ, Alvarez GS, Martins PDE, Oliveira MP. Uso de retalho cutâneo para reconstrução nasal após ressecção neoplásica: 102 casos. Rev Bras Cir. Plást. 2012;27(3):21.
51. Severo Júnior LCV, Chambô F, Dibe MJA, Leal PRA. Retalho miocutâneo dorsoglabelar baseado na artéria nasal lateral para reconstrução de defeitos da ponta nasal. Arq Catarin Med. 2007;36(Supl 1):124-7.
52. Paiva GR, Bidart JL, Rocha S. Retalho musculocutâneo nasal ilhado para reconstrução de defeitos do nariz. Rev Bras Cir Plást. 2009;24(2):182-94.
53. Namiuchi NM, Ledo-Silva MC, Safaddini E, Oliveira EL, Beringer M, Abramo AC. Retalhos cutâneos de avanço de ambas hemifaces para reconstrução do nariz após múltiplos carcinomas basocelulares no dorso nasal. Rev Bras Cir Plást. 2009;24(4):563-5.
54. Jaeger MRO, Amaral Neto N, Silva JB. Reparação dos defeitos parciais do nariz após excisão tumoral. Rev AMRIGS. 2011;55(1):83-7.
55. Tissiane LAL, Alonso N, Carneiro MH, Bazzi K, Rocco M. Versatilidade do retalho bilobado. Rev Bras Cir Plást. 2011;26(3):411-7.
56. Chren MM, Sahay AP, Sands LP, Maddock L, Lindquist K, Bertenthal D, et al. Variation in care for nonmelanoma skin cancer in a private practice and a veterans affairs clinic. Med Care. Med Care. 2004;42(10):1019-26. DOI: http://dx.doi.org/10.1097/00005650-200410000-00011
57. Essers BA, van Helvoort-Postulart D, Prins MH, Neumann M, Dirksen CD. Does the inclusion of a cost attribute result in different preferences for the surgical treatment of primary basal cell carcinoma?: a comparison of two discrete-choice experiments. Pharmacoeconomics. 2010;28(6):507-20. DOI: http://dx.doi.org/10.2165/11532240-000000000-00000
Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
Institution: Clinica Wulkan, São Paulo, SP, Brazil.
Corresponding author:
Marcelo Wulkan
Rua Batatais, 309, Jd. Paulista
São Paulo, SP, Brazil Zip Code 01423-010
E-mail: mawulkan@yahoo.com
Article received: January 5, 2014.
Article accepted: August 3, 2014.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter