

Case Report - Year 2015 - Volume 30 -
Hidradenitis suppurativa (acne inversa): review of the literature and case report on the surgical treatment of a presternal lesion
Hidradenite supurativa (acne inversa): revisão da literatura e relato de caso sobre o tratamento cirúrgico de lesão pré-esternal
ABSTRACT
Hidradenitis suppurativa is a chronic debilitating and stigmatizing disease that is difficult to treat. The disease presents several clinical characteristics, which may occur alone or simultaneously in various locations, generally symmetrical and distributed in the "milk line". It affects the following areas of the skin where intertriginous apocrine glands are numerous, in the descending order: axilla, anogenital region, areolas, and inframammary crease. Its insidious progression begins with formation of subcutaneous nodules that rupture and/or coalesce, forming extremely painful abscesses in the deep dermis. The lesions often drain foul purulent exudate, with significant damage to quality of life. As the disease progresses, formation of fistulas, comedones, fibrosis, dermal contractures, and hardening of the skin occur. The highest chances of cure are lie in early diagnosis and individualized treatment, which covers pharmacological, behavioral, and surgical measures. Surgical treatment has been considered a more effective curative measure. The decision between the different modalities will depend on the stage, presentation, and local commitment and include incision and drainage of abscesses, deroofing, marsupialization, electrosurgery, Nd:YAG laser, CO2 laser, and extensive surgical excision. The reconstruction options include healing by second intention, immediate or delayed full-thickness skin graft, primary closure, and flaps. The reported case of presternal injuries presented clinical and histological characteristics compatible with hidradenitis suppurativa; this location has been rarely reported in the literature. The postoperative results of complete resection of the lesion with primary closure indicated resolution over a long follow-up period. More randomized clinical trials are needed to determine the best management strategy for hidradenitis suppurativa.
Keywords: Hidradenitis suppurativa; Sternum/injuries; Reconstructive surgical procedures.
RESUMO
A Hidradenite Supurativa é uma doença crônica debilitante, estigmatizante e de difícil tratamento. A doença apresenta várias características clínicas, podendo ocorrer isolada ou simultaneamente em diversas localizações, geralmente simétricas, distribuídas na "linha do leite". Afeta a pele onde há maior quantidade de glândulas apócrinas intertriginosas, em ordem decrescente: axilas, região ano-genital, aréolas e sulco inframamário. Seu curso insidioso inicia com nódulos subcutâneos que se rompem e/ou coalescem, formando abscessos na derme profunda, extremamente doloridos. As lesões frequentemente drenam exudato purulento fétido, com importante prejuízo à qualidade de vida. Com a progressão da doença, ocorre formação de fistulas, comedões, fibrose, contraturas dérmicas e endurecimento da pele. Suas maiores chances de cura estão no diagnóstico precoce e tratamento individualizado, que abrange medidas farmacológicas, comportamentais e cirúrgicas. O tratamento cirúrgico tem sido considerado a medida curativa mais efetiva. A decisão entre as diversas modalidades vai depender do estágio, apresentação e comprometimento local e incluem incisão e drenagem dos abscessos, deroofing, marsupialização, eletrocirurgia, laser Nd:YAG, laser de CO2 e excisão cirúrgica extensa. As opções de reconstrução incluem cicatrização por segunda intenção, enxerto de pele total imediato ou tardio, fechamento primário e retalhos. O caso relatado de lesões préesternais apresentava características clínicas e histológicas compatíveis com HS, sendo esta uma localização incomum na Literatura. O resultado pós-operatório da ressecção de toda a lesão com fechamento primário mostrou-se resolutivo após longo tempo de seguimento. Mais ensaios clínicos randomizados são necessários para estipular o melhor manejo na HS.
Palavras-chave: Hidradenite supurativa; Esterno/lesões; Procedimentos cirúrgicos reconstrutivos.
Hidradenitis suppurativa (HS) is a chronic and recurrent suppurative skin disease, also known as "acne inversa." Its insidious development begins with subcutaneous nodules that rupture and coalesce, forming extremely painful abscesses in the deep dermis. These abscesses often drain a foul purulent exudate, causing significant harm to personal relations. With the progression of the disease, fistulas, comedones, fibrosis, dermal contractures, and skin hardening occur1-9.
The disease presents several clinical characteristics, which may occur alone or simultaneously in various locations, generally symmetrical and distributed in the "milk lines." The lesions more commonly involve the axilla, groin, anogenital region, and inframammary crease, among others1,2,4,6-10.
The objective of this article is to present a rare case of HS in the presternal region and present a review of the literature on HS, focusing on surgical treatment.
CASE REPORT
A 22-year-old female patient was referred by the infectologist for evaluation of drainage of purulent secretion in the sternal region. Two years prior, she underwent laparoscopic sympathectomy for bilateral axillary hyperhidrosis. She developed paradoxical sweating in the dorsal regions, chest, and upper abdomen. After 12 months, inflammatory nodules appeared in the inguinal and presternal region. The nodules in the inguinal region were resistant to clinical treatment with antibiotic therapy. According to the clinical presentation, a diagnosis of HS was made. The left inguinal region displayed mixed flora on bacterioscopy. The lesion was resolved with amoxicillin/clavulanate administration. The presternal lesion, already at 1.5 years of development, exhibited intermittent drainage of a purulent non-fetid secretion in several points and intense pain.
Upon examination, erythematous irregular nodular lesions with multiple transdermal fistulas were observed, with elimination of small amounts of non-fetid, thick, and yellow secretion. The average greater length of the lesion was about 10 cm, especially longitudinal in the presternal region. Ultrasonography revealed an anechoic nodular formation (collection) with increased peripheral vasculature, regular contours measuring 1.0 cm in diameter, and subcutaneous topography anterior to the body of the sternum but without evidence of bone injury. Nuclear magnetic resonance revealed no osteomyelitis in the sternum. Bacteriological examination revealed mixed flora and growth of coagulase-negative staphylococci. Inflammatory markers were negative, and the preoperative laboratory test results were normal.
METHODS
Because of the persistent and long period of unsuccessful conservative management (1.5 years), the author, along with the patient, opted for surgery. Wide resection of soft tissues was performed in a single block in the presternal region, with 2 cm lateral margins and a depth up to the supraperiostal plane (Figures 1 to 4), with approximation of margins and maintenance of a catheter for washing (Figure 5).
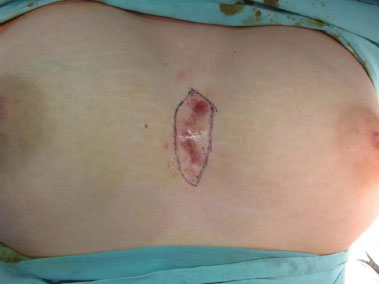
Figure 1. Preoperative aspect.
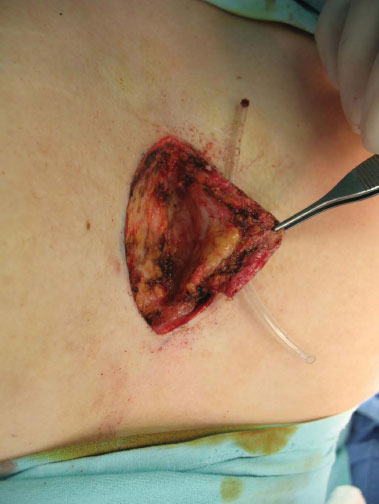
Figure 2. Surgical excision after catheterization of the orifice with resection up to the limit of the periosteum.
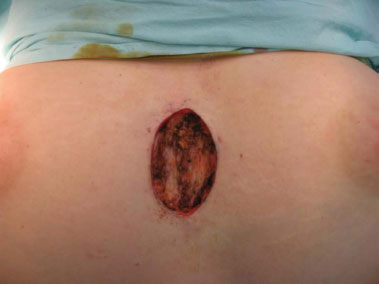
Figure 3. Intraoperative aspect.
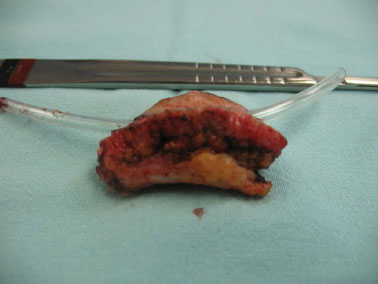
Figure 4. Surgical specimen showing the anatomical planes.
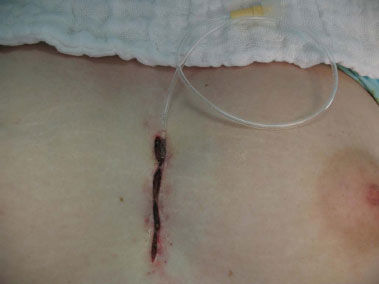
Figure 5. Postoperative aspect with an aspiration tube for later intermittent flushing with saline for 7 days.
RESULTS
Good postoperative evolution was observed, without wound infection or dehiscence. No impairment of the periosteum was observed on macroscopic examination. The anatomopathological diagnosis indicated acute and chronic suppurative inflammation with formation of microabscesses fistulized in the skin, compatible with HS. During 3 years of postoperative follow-up, the patient had 3 episodes of folliculitis in a single point, with spontaneous regression and without formation of abscesses or cutaneous fistula in the presternal intermammary region (Figures 6 and 7).
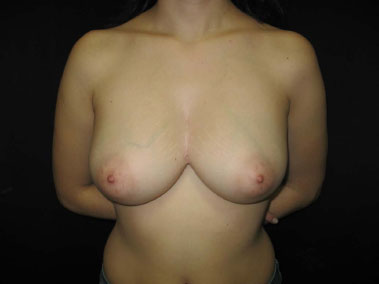
Figure 6. Three-year postoperative aspect.
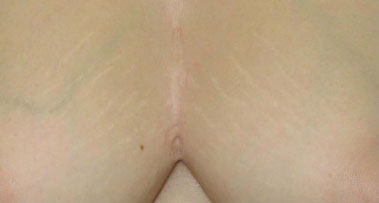
Figure 7. Three-year postoperative aspect: close.
DISCUSSION AND LITERATURE REVIEW
Definition and Diagnostic Criteria
Initially described by Verneuil in 1854, the etiopathogenesis of HS was assigned to a disorder of apocrine sweat glands, hence the name "hidradenitis"5,7,9,11,12. The perception that the main event in the etiology of HS involves the hair follicle, as an acneiforme disorder, and not the apocrine glands, led to the suggestion of the term acne inversa1-3,5-9,11.
The Second International Symposium of Hidradenitis Suppurativa9 adopted in 2009 the following definition: "HS is a chronic inflammatory, recurrent, debilitating follicular disease that usually manifests after puberty with painful inflamed lesions of apocrine glands, most commonly axilla and axillary and inguinal regions." The diagnostic criteria include the following: typical lesions (painful nodules, abscesses, sinus tracts, scarring bands, and comedones), typical topography (axilla, groin and perianal area, buttocks, and inframammary fold), chronicity, and recurrence. The presence of three criteria establishes the diagnosis. These lesions usually present a late diagnosis and inadequate management, often diagnosed as furuncles.
Epidemiology
The peak incidence occurs between the age of 11 and 30 years (second and third decades of life), in both sexes, but women are more affected at a proportion of 3:1. The exact prevalence is not known and has been estimated at 1 per 100 persons to 1 per 600 persons. Compared to axillary lesions, perineal lesions are associated with higher recurrence rates and unfavorable development1,9,13,14. Axillary lesions are predominant in female patients; and perineal lesions, in male patients12.
Etiopathogenesis
The initial predisposing factors that lead to follicular obstruction have not yet been clarified, but are most likely multifactorial. The condition seems to be a disease of the follicular epithelium rather than a disease of apocrine glands. A disorder of the complex mechanism involving receptors, immunomodulators, and genes may be involved in the cascade of events that lead to the infundibular obstruction1-2,5-9,12. Secondary bacterial colonization can exacerbate the disease, but it is not a primary etiological factor because the cultures are often sterile and antibiotics are not curative. The bacteria most commonly involved include Staphylococcus aureus, Streptococcus epidermidis, Streptococcus milleri, Streptococcus viridans, Chlamydia trachomatis, anaeróbios (Peptostreptococcus, Bacteroides, and Fusobacterium), Escherichia coli, Klebsiela, and Proteus. The last four are more common in perineal HS. The most common bacteria are Staphylococcus aureus and Streptococcus epidermidis1-3,6-9.
The etiological factors strongly associated with HS are smoking and obesity, but are not primary causes of the disease. The smoking patient experiences a more aggressive form of the disease, which may even disappear with the cessation of smoking. This mechanism remains unknown but seems to be multifactorial. The nicotine present in tobacco smoke plays a role in the stimulation and dysfunction of glandular secretion, in addition to changing the chemotaxis of neutrophils. Obesity can aggravate HS through the retention of sweat, skin maceration, rupture of follicular and glandular ostia, and a change in hormonal metabolism, factors that predispose to occlusion of pores1,2,5-9. Immune disorders, hyperandrogenic conditions, genetic predisposition (familiar), use of oral contraceptives, lithium, and chemical irritants such as antiperspirants and deodorants are still under investigation as causal factors1-2,5-7,9,12,15. The effect of hormonal factors is controversial, but recent studies indicate a strong relationship between sex hormones and HS. However, more studies are required.
Clinical Presentation and Natural History of the Disease
The diagnosis is essentially clinical with the following typical criteria: insidious lesions in flexural areas with apocrine glands, poor response to antibiotics, and strong tendency to recur. It affects areas of the skin where there are more intertriginous apocrine glands, the most common being the axilla, groin, anogenital region, pubis region, periareolar region, inframammary fold, buttocks, periumbilical region, scalp, and retroauricular region, among others1,2,4,6-10. Atypical presentations include urethral skin fistula, nipple fistulae, and extensive lumbosacral epidural abscesses1,2,4.
The Hurley classification1,11,12,16,17 divides patients into the following 3 groups according to the presence and extent of cicatrization and sinus tracts: stage I, formation of single or multiple abscesses, without sinus tracts or scar; stage II, one or more recurring abscesses with formation of sinus tracts and scars; and stage III, multiple interconnected sinuses with abscesses across the entire area.
The initial symptoms are pain, pruritus, erythema, and hyperhidrosis, with hardened erythematous nodules or cysts that rupture, releasing foul purulent material. The prodromes (itching, burning, pain, and hyperhidrosis) are perceived by 50% of patients 12 to 48 hours before the appearance of nodules. Recurrent abscesses that do not respond to standard antibiotic therapy should indicate HS9. The areas that cicatrize become fibrotic, but new lesions may appear around it. The clinical picture is characterized by several recurrences and may evolve to ever increasing areas with the formation of sinus tracts with secretion, double comedones (typical of the disease), and infected and painful inflammatory masses. The lesions usually drain spontaneously, with formation of multiple sinus tracts and hypertrophic scar. The deep abscesses break horizontally under the skin, forming subcutaneous paths that lead to scars and suppuration. The final stage is characterized by chronic recurrence, with subcutaneous abscesses and sinus tracts1-3,6,7,9,16. These signs occur normally in the axilla and anogenital region, with remarkable symmetry. Severe cases develop in depth, reaching muscles, fascia, and intestines1-3,6-9. Exacerbations can occur by stress, heat, sweat, and tight clothing7. Patients with HS can also be affected with acne, pilonidal cysts, and chronic scalp folliculitis, a condition known as follicular occlusion tetrad1,3,12.
This chronic inflammatory process may be involved in the onset of complications such as non-melanocytic skin cancer, contractures and limitation of limb mobility, arthropathy, osteomyelitis, severe infections, recurrent cellulitis, lymphedema distal to scars, stenosis and/or anal/urethral fistula, lymphangiectasia, amyloidosis, reflex sympathetic dystrophy, toxic shock syndrome, acute hypokalemia, hypoproteinemia, and anemia. The most patients with severe cases become isolated, depressive, and suicidal1,2,9,11,12.
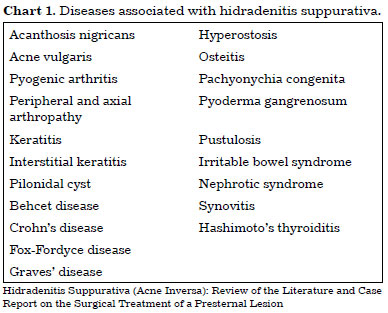
HS can be associated with diseases such as acne vulgaris, pilonidal cyst, and Crohn's disease (Chart 1)1-4,12,15. This condition correlated with Marjolin's ulcer in some reports, almost always with delayed referral to surgeons18-21. The greater possibility of malignant transformation in these patients22 ensures the need for sending the surgical specimen and any suspected lesion for pathological examination21. The patients should undergo serial clinical examinations of lesions.
An important point to be emphasized is that HS is very debilitating, physically and psychologically. The drainage and odor of the abscesses leak onto clothes, causing social embarrassment. Recent studies conclude that patients with HS have a worse quality of life than those with other conditions such as chronic urticaria, psoriasis, and neurofibromatosis. The assessments are negatively influenced by pain, number of lesions, severity, and progressive nature. HS can lead to depression, fatigue, stigmatization, and reduced productivity. Patients with severe clinical presentations refrain from socializing, culminating in economic and social harm1,2,8,12.
Differential Diagnosis
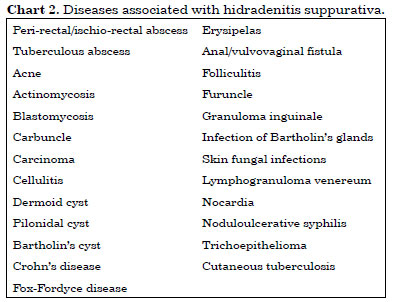
HS must be differentiated from conditions such as furunculosis, carcinoma, and Crohn's disease (Chart 2)1,4,6-9,15,23. The differentiation is based on clinical history, appearance, age at onset, typical location, and histological examination1,2. The most common differential diagnosis involves folliculitis, furuncles, and carbuncles. Acne produces similar lesions but with different distributions. The often-unspecific characteristics of HS lead to delayed diagnosis. The present patient presented her problem to several experts, none of whom, however, had established a diagnosis. Diagnosis may be delayed for 7 years on an average and may even be delayed for decades9.
Treatment
HS is a skin disease that is difficult to treat, and treatment success rates are dependent on the stage of the disease and the therapeutic approach1,3,16. An adequate early and multidisciplinary approach offers the best possibility of controlling the disease.6 Treatment involves preventive, clinical, surgical, and psychological measures.
Once the diagnosis is made, the severity and location of the lesions should be evaluated so that the appropriate treatment modality may be used individually, in accordance with the circumstances and preferences of the patient.
Clinical Treatment
Antibiotics are the main treatment agents in the early stages (Hurley I and II), when they may relieve the symptoms. The initial empirical therapy should cover Staphylococcus aureus, as well as potential aerobic and anaerobic pathogens. Long-term topical antibiotic therapy can be administered with clindamycin. Clindamycin, rifampicin, metronidazole with beta-lactamase-resistant penicillin, clavulanic acid and amoxicillin, and cefoxitin, among others, are indicated systemically against Staphylococcus aureus and anaerobic organisms1,3,6-8,12. A recent systematic review of 62 studies on HS17 indicated the use of topical clindamycin 1% for Hurley stage I cases. For Hurley stage II, systemic clindamycin and rifampicin can be initiated for a long period. In both stages, one must also consider the use of Nd:YAG laser monthly. Antibiotics are not curative, and this treatment alone presents high rates of recurrence, although it reduces the purulent discharge and pain. Other measures that are still under study include hormonal therapies, steroids, immunosuppressive agents and inhibitors of TNF-alpha, photodynamic therapy, cryotherapy, anti-inflammatory agents, radiotherapy, and retinoids1,2,6-8,11,12,17.
Non-adherent dressings must be used in accordance with the conditions of the wound. Recommendations for all patients include education about the chronic and relapsing nature of the disease, cessation of cigarette smoking, and weight loss. Measures without clinical trial evidence include avoiding tight clothes, exposure to heat, skin irritants (shaving and deodorant use), and dealing with stress. Regular monitoring with a multidisciplinary support group is essential, with the cooperation of surgeons, clinicians, psychologists, physiotherapists, and specialized staff in the management of wounds1,6-8,17.
Surgical Treatment
Surgical treatment has been considered as the only effective curative measure. In general, advanced stages benefit mainly from surgery12. In a recent systematic review, Rambhatla et al.17 indicated that patients with refractory and severe disease must be referred to surgeons to discuss surgical options. The decision between the various arrangements will depend on the stage, severity, and local commitment. As spontaneous resolution is rare and the progression of the disease is highly likely, early surgical treatment is advisable. Initial conservative measures such as incision and floating drainage of abscesses can relieve acute symptoms but with high rates of recurrence1,2,6-8. Debridement (deroofing) and marsupialization are simple procedures and, together with ablative procedures such as electrosurgery, Nd:YAG laser, or CO2 laser, are indicated in early and limited cases (stages I and II Hurley)1-3,6,7,16. In chronic and relapsing disease, the primary treatment is extensive surgical excision throughout the affected area with free margins, generally adequate between 1 to 2 cm12. Studies show that the extent of resection is of great importance in reducing recurrence rates compared with clinical care of wounds7,8. Limited excisions present a higher risk of recurrence than extensive excisions12,17.
Many options have been discussed for reconstruction after excision of lesions. Options include healing by second intention, immediate or delayed full-thickness skin grafting, and primary closure and grafting. Closure by secondary intention requires a longer period of recovery and meticulous care of the wound, and may evolve with good esthetic results. However, conflicting results have been reported regarding recurrence after this technique. Grafts have been widely used, with the disadvantages of scar formation in the donor site, contractures, and lower esthetic quality12.
Some authors advocate excision and primary closure for localized axillary disease, citing less morbidity, fewer complications, and shorter length of hospital stay than those in more-extensive procedures. One can achieve excellent results in experienced hands through primary closure. The high recurrence rates initially assigned to primary closure are possibly in fact due to compromised margins by incomplete resections and/or the natural course of the disease7,8,10,12,17,24,25.
Fasciocutaneous and musculocutaneous flaps have been increasingly used in reconstruction in HS. The following options classically used in reconstructive plastic surgery are used: Limberg flap, pedicled flap in lateral thoracic or circumflex scapular arteries, posterior arm fasciocutaneous flap, and latissimus dorsi flap. As an example of a reconstruction material for inguinal-perineal lesions, one can mention the gracilis flap; for inguinal and vulval lesions, the bilateral V-Y flaps based on arterial perforators of the gluteus maximus and anterolateral thigh flap; and for perianal lesions and below the gluteus, the musculocutaneous flap from the upper half of the gluteus maximus10,12,25. Despite the significant number of techniques described, randomized clinical trials that establish the recurrence rates between the different surgical options are still lacking.
The prognosis differs between patients, which may vary from moderate forms with benign progression to chronic cases, and with recurrence and years of duration. In these cases, the progression to surgical treatment is common, in which a radical excision is usually indicated7.
In this case, after 6 months of presternal surgery, the patient presented recurrence of the left inguinal and axillary HS. Both were treated surgically, without recurrence up to the present time.
The patient developed compensatory hyperhidrosis in the presternal region after sympathectomy, a previously asymptomatic region. This may have triggered the HS. The literature describes some dermatological diseases that accompany hyperhidrosis, such as dyshidrosis, Frey's syndrome, and HS itself. Other diseases may also be aggravated by excessive sweating, such as inverse psoriasis and aquagenic palmoplantar keratoderma26. O'Reilly et al.27 suggest this association because of the fact that the botulinum toxin is effective in the treatment of HS, based on the reduction of the production of apocrine sweat, thus limiting the tendency for follicular rupture. Still, Ather et al. cite sweating as a cause of exacerbation of HS crisis7,28. This causal relationship and treatment method require further research in the future.
Bacteriology indicated Staphylococcus aureus, compatible with the most commonly involved bacteria. Treatment with antibiotics alone was not curative, in agreement with previous reports1-4,10,16. Surgery was indicated in view of resistance to clinical treatment, opting for primary closure.
Surgical treatment consisted of sufficiently free excision margins to reduce the chance of recurrence, with resection of the entire affected area. In the 3-year follow-up, no recurrences were found in this region.
CONCLUSION
HS is a chronic disease with controversial etiology and management. An early multidisciplinary approach must be determined individually. In this report, the postoperative outcome of an extensive surgical resection of a presternal lesion with primary closure was resolved after 3 years of follow-up. More randomized clinical trials are needed to determine the best management strategy for HS.
REFERENCES
1. Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60(4):539-61. DOI: http://dx.doi.org/10.1016/j.jaad.2008.11.911
2. Buimer MG, Wobbes T, Klinkenbijl JH. Hidradenitis suppurativa. Br J Surg. 2009;96(4):350-60. PMID: 19283748 DOI: http://dx.doi.org/10.1002/bjs.6569
3. Brook I. The role of anaerobic bacteria in cutaneous and soft tissue abscesses and infected cysts. Anaerobe. 2007;13(5-6):171-7. DOI: http://dx.doi.org/10.1016/j.anaerobe.2007.08.004
4. Cosman BC, Al-Refaie WB. Mammillary fistula as a manifestation of acne inversa (hidradenitis suppurativa): report of two cases. J Am Coll Surg. 2002;194(6):829-33. DOI: http://dx.doi.org/10.1016/S1072-7515(02)01141-9
5. Sellheyer K, Krahl D. What causes acne inversa (or hidradenitis suppurativa)?--the debate continues. J Cutan Pathol. 2008;35(8):795-7. PMID: 18687077 DOI: http://dx.doi.org/10.1111/j.1600-0560.2008.01108.x
6. Velasco AL, Dunlap WW. Pilonidal disease and hidradenitis. Surg Clin North Am. 2009;89(3):689-701. PMID: 19465205 DOI: http://dx.doi.org/10.1016/j.suc.2009.02.003
7. Ather S, Chan DS, Leaper DJ, Harding KG. Surgical treatment of hidradenitis suppurativa: case series and review of the literature. Int Wound J. 2006;3(3):159-69. DOI: http://dx.doi.org/10.1111/j.1742-481X.2006.00235.x
8. Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24(2):118-23. DOI: http://dx.doi.org/10.1097/QCO.0b013e3283428d07
9. Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28(4):779-93. DOI: http://dx.doi.org/10.1016/j.det.2010.07.003
10. Rees L, Moses M, Clibbon J. The anterolateral thigh (ALT) flap in reconstruction following radical excision of groin and vulval hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2007;60(12):1363-5. DOI: http://dx.doi.org/10.1016/j.bjps.2007.08.013
11. Trombetta M, Werts ED, Parda D. The role of radiotherapy in the treatment of hidradenitis suppurativa: case report and review of the literature. Dermatol Online J. 2010;16(2):16.
12. Ellis LZ. Hidradenitis suppurativa: surgical and other management techniques. Dermatol Surg. 2012;38(4):517-36. DOI: http://dx.doi.org/10.1111/j.1524-4725.2011.02186.x
13. Thornton JP, Abcarian H. Surgical treatment of perianal and perineal hidradenitis suppurativa. Dis Colon Rectum. 1978;21(8):573-7. PMID: 738172 DOI: http://dx.doi.org/10.1007/BF02586399
14. Williams ST, Busby RC, DeMuth RJ, Nelson H. Perineal hidradenitis suppurativa: presentation of two unusual complications and a review. Ann Plast Surg. 1991;26(5):456-62. DOI: http://dx.doi.org/10.1097/00000637-199105000-00008
15. Hsiao JL, Antaya RJ, Berger T, Maurer T, Shinkai K, Leslie KS. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146(11):1265-70. PMID: 21079064 DOI: http://dx.doi.org/10.1001/archdermatol.2010.328
16. Aksakal AB, Adişen E. Hidradenitis suppurativa: importance of early treatment; efficient treatment with electrosurgery. Dermatol Surg. 2008;34(2):228-31. DOI: http://dx.doi.org/10.1097/00042728-200802000-00013
17. Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148(4):439-46. PMID: 22184715 DOI: http://dx.doi.org/10.1001/archdermatol.2011.1950
18. Altunay IK, Gokdemir G, Kurt A, Kayaoglu S. Hidradenitis suppurativa and squamous cell carcinoma. Dermatol Surg. 2002;28(1):88-90. PMID: 11991278
19. Crain VA, Gulati S, Bhat S, Milner SM. Marjolin's ulcer in chronic hidradenitis suppurativa. Am Fam Physician. 2005;71(9):1652. PMID: 15887445
20. Lin MT, Breiner M, Fredricks S. Marjolin's ulcer occurring in hidradenitis suppurativa. Plast Reconstr Surg. 1999;103(5):1541-3. PMID: 10190470
21. Katz RD, Goldberg NH. Marjolin ulcer arising within hidradenitis: a case report and literature review. Ann Plast Surg. 2009;62(2):173-4. PMID: 19158529 DOI: http://dx.doi.org/10.1097/SAP.0b013e31817d87b3
22. Lapins J, Ye W, Nyrén O, Emtestam L. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137(6):730-4. PMID: 11405761
23. Kamada A, Saga K, Jimbow K. Apoeccrine sweat duct obstruction as a cause for Fox-Fordyce disease. J Am Acad Dermatol. 2003;48(3):453-5. DOI: http://dx.doi.org/10.1067/mjd.2003.93
24. Banerjee AK. Surgical treatment of hidradenitis suppurativa. Br J Surg. 1992;79(9):863-6. PMID: 1422743 DOI: http://dx.doi.org/10.1002/bjs.1800790905
25. Dabernig J, Sorensen K, Shaw-Dunn J, Hart AM. The thin circumflex scapular artery perforator flap. J Plast Reconstr Aesthet Surg. 2007;60(10):1082-96. DOI: http://dx.doi.org/10.1016/j.bjps.2006.10.002
26. Messikh R, Atallah L, Aubin F, Humbert P. Botulinum toxin in disabling dermatological diseases. Ann Dermatol Venereol. 2009;136 Suppl 4:S129-36. DOI: http://dx.doi.org/10.1016/S0151-9638(09)74540-5
27. O'Reilly DJ, Pleat JM, Richards AM. Treatment of hidradenitis suppurativa with botulinum toxin A. Plast Reconstr Surg. 2005;116(5):1575-6. PMID: 16217533 DOI: http://dx.doi.org/10.1097/01.prs.0000184354.32111.dc
28. von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14(5):389-92. DOI: http://dx.doi.org/10.1046/j.1468-3083.2000.00087.x
Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil
Institution: Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil.
Corresponding author:
Milton Paulo de Oliveira
Rua Ramiro Barcelos, 1172, Moinhos de Vento
Porto Alegre, RS, Brazil Zip Code 90035-006
E-mail: miltonpaulo.poa@gmail.com
Article received April 2, 2012.
Article accepted July 23, 2012.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter