

Original Article - Year 2015 - Volume 30 -
Breast reconstruction in two stages with tissue expanders and silicone breast implants
Reconstrução mamária em dois estágios com expansores de tecido e implantes de silicone
ABSTRACT
INTRODUCTION: Immediate breast reconstruction with an expander/implant is a good option for women submitted to mastectomy. This study aimed to evaluate the results of immediate breast reconstruction with implants and expanders in patients who did or did not undergo postoperative radiotherapy.
METHODS: A consecutive prospective study that involved 83 women submitted to immediate breast reconstruction, was carried out by first performing breast reconstruction surgery with expanders and then with implants. The study was conducted between 2007 and 2012 and accounted for a total of 90 reconstructions. In the first surgery, an expander was placed in a submuscular bag under the pectoralis major and serratus muscles. In the second surgery, the expander was replaced by an implant. We compared the surgical outcomes of both types of surgeries (with implants and with expanders) in patients who underwent postoperative radiotherapy and those who did not.
RESULTS: After the first surgery, 33 patients (39.8%) received radiotherapy, and 13.25% experienced complications, including expander displacement (4.8%), emptying (2.4%), infection (2.4%), skin dehiscence (2.4%), and extrusion (1.2%). After the second surgery, 17.6% of the subjects experienced complications, including capsular contracture (7%), extrusion (5.3%), and infection (5.3%). With regard to the first surgery, 18.8% of the patients who underwent radiotherapy and 10.0% of those who did not, experienced complications. With regard to the second surgery, these prevalences were 46.6 % and 7.14%, respectively.
CONCLUSION: Patients who received radiotherapy after breast reconstruction surgery experienced more complications.
Keywords: Breast cancer; Mammoplasty; Tissue expanders; Breast implants; Radiotherapy.
RESUMO
INTRODUÇÃO: A reconstrução mamária imediata com expansor/implante permanece uma opção importante para mulheres submetidas à mastectomia. O objetivo desse estudo foi avaliar os resultados de reconstrução mamária imediata com emprego expansores e implantes em pacientes submetidas à radioterapia e não submetidas à radioterapia no pós-operatório.
MÉTODOS: Foi realizado estudo prospectivo consecutivo com 83 mulheres submetidas à reconstrução mamária imediata com expansores seguido de implantes no período de 2007 a 2012, totalizando 90 reconstruções. No primeiro tempo cirúrgico, o expansor foi colocado em uma bolsa submuscular sob os músculos peitoral maior e serrátil. No segundo tempo, o expansor foi substituído pela prótese. Dois grupos de pacientes foram comparados: (1) pacientes que receberam radioterapia no pós-operatório e (2) pacientes que não receberam radioterapia no pós-operatório. Os resultados foram avaliados em cada grupo nos dois tempos cirúrgicos.
RESULTADOS: Trinta e três pacientes receberam tratamento radioterápico (39,8%) no final da expansão. Observamos a ocorrência de 13,25% de complicações no primeiro tempo: deslocamento do expansor (4,8%), esvaziamento (2,4%), infecção (2,4%), deiscência de pele (2,4%) e extrusão (1,2%). No segundo tempo, as complicações foram 17,6%: contratura capsular (7%), extrusão (5,3%), e infecção (5,3%). Com relação aos grupos estudados no primeiro tempo, aqueles que fizeram radioterapia tiveram (18,18%) de complicações e o grupo não exposto à radioterapia apenas (10,0%). No segundo tempo, encontramos 7 (46,6%) complicações para radioterapia e apenas 3 (7,14%) para o grupo não irradiado.
CONCLUSÕES: Pacientes que receberam radioterapia posteriormente à reconstrução apresentaram maiores índices de complicações.
Palavras-chave: Câncer de mama; Mamoplastia; Expansores de Tecido; Implantes de Mama; Radioterapia.
Breast cancer is the most common type of cancer occurring in women and accounts for 22% of new cancer cases per year. In Brazil, the mortality rates are high. Worldwide, the average survival rate after five years of cancer detection is 61%. According to the World Health Organization, there was a 10-fold increase in the cancer incidence rate in the 1960s and 1970s1.
The indications for breast reconstruction have been affected by the changes in the surgical treatment of breast cancer. In the late 20th century, prospective randomized studies showed that survival rates were not related to the type of surgery performed, allowing the use of conservative treatments for breast cancer in the early stages of the disease2,3.
The psychological benefit and oncologic safety of immediate breast reconstruction are already well established. Women who undergo this procedure regain their breast physical contour, recover their self-esteem, and feel unmutilated4.
Immediate breast reconstruction using expanders and implants became a common procedure following the introduction of tissue expanders in breast reconstruction5.
With the increasing use of radiotherapy (RT) as part of a multimodal approach in the treatment of breast cancer, plastic surgeons are increasingly faced with patients having a history of previous radiation or requiring radiotherapy after mastectomy seeking breast reconstruction.
Despite the proven oncologic safety of mastectomy associated with immediate reconstruction, we questioned the interference of adjuvant treatments, such as RT, on the reconstructed breast.
OBJECTIVE
This study aimed to evaluate the results of immediate breast reconstruction with implants and expanders in patients submitted to RT, and in those who were not exposed to RT in the postoperative period.
METHODS
We conducted a prospective, consecutive study of 83 women with breast cancer who underwent two surgeries for immediate breast reconstruction between May 2007 and December 2012; the first using breast expanders and the second using breast silicone breast implants. Of 83 patients, 7 were submitted to bilateral mastectomy, resulting in a total of 90 reconstructed breasts. The age of patients ranged from 22 to 70 years, with an average age of 46.8 years. Patients with decompensated systemic diseases, advanced and inflammatory tumors, and/or who were previously submitted to breast RT were not included in the study. The patients were referred from outpatient medical centers and private clinics and all breast reconstructions were performed by the same surgeon.
The volume of the expanders was calculated on the basis of the thoracic width, contralateral breast volume, and patient preference. The expander volume ranged from 200.0 ml to 600.0 ml, with an average of 407.7 ml.
Reconstruction with an expander was performed immediately after the mastologist performed the mastectomy. The expander was inserted after a submuscular bag was created under the pectoralis major and serratus anterior muscles, thus allowing the expander to be fully covered by muscle (Figure 1).
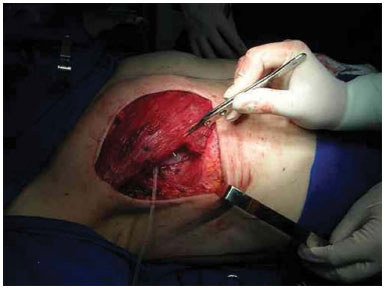
Figure 1. Expander positioned in the submuscular bag.
The valves were positioned in the lateral thoracic region through a subcutaneous tunnel6. Vacuum suction drains were inserted in all patients. All patients received antibiotic therapy (cefadroxil 500 mg every 12 h) for 7 days.
Intraoperative expansion with 10% expander volume was performed in all patients. The average duration of the surgery was 55 min.
The expansion sessions were initiated in the fourth postoperative week and continued till the total volume desired before RT was obtained; due consideration was given to the contralateral breast size and patient concerns (Figure 2). Each week, the breasts were enlarged by 20% of the expander volume. The average time between the first and second surgeries was 15 months (range: 7 m to 4 y). The second stage of the reconstruction included the removal of the tissue expander, a capsulotomy and capsulectomy, repositioning of the inframammary fold (if necessary), and placement of permanent implants. During this time, symmetrization of the contralateral breast was also performed (Figures 2, 3, 4, 5, and 6).
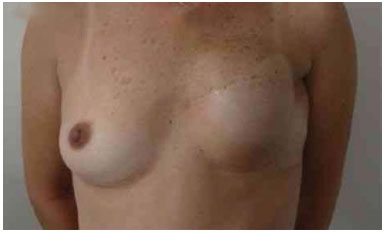
Figure 2. Postoperative period of 6 months after reconstruction with the expander. Full expansion with 380 ml.
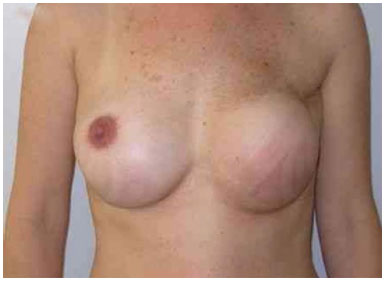
Figure 3. Postoperative period of 1 year after replacement of the expander by the implant, repositioning of the inframammary fold, and symmetrization of the right breast with the implant.
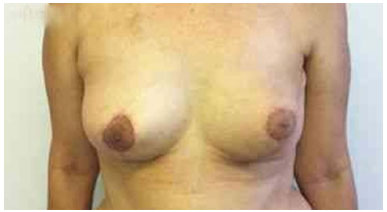
Figure 4. Postoperative period of 90 days after replacement of the expander by the implant in the left breast and symmetrization of the right breast.
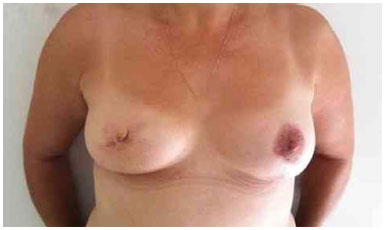
Figure 5. Postoperative period of 1 year after replacement of the expander by the implant in the right breast and symmetrization of the left breast.
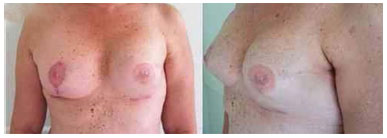
Figure 6. Postoperative period of 4 months after replacement of the expander by the implant in the left breast and symmetrization of the right breast.
RESULTS
We evaluated 83 patients who submitted to 90 immediate postmastectomy breast reconstructions within the study period (from March 20, 2007, to December 12, 2012). Of these, 33 (39.8%) were submitted to postoperative RT, whereas 50 (60.2%) did not receive RT. Their average age was 46.8 years (standard deviation [SD] = 10.5). In Graph 1, we plotted the age distribution among individuals who did or did not receive RT in the postoperative period after breast reconstruction. The average age of the group that did not receive RT was 47.7 y (SD = 9.8), and that of the group that received RT was 45.6 years (SD = 11.4). In total, 91% of those who received RT were older than 35 years, 12.12%, 20-35 years; 57.58%, 35-50 y; and 30.30%, > 50 y. Of those who did not receive RT, 8% were 20-35 years old, 48% were 35-50 years old, and 44% were > 50 years (Figure 7).
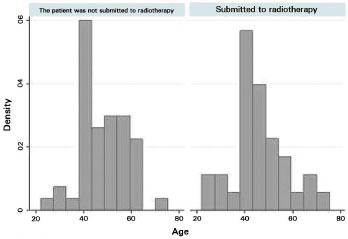
Figure 7. Age histograms among individuals submitted to breast reconstruction with or without postoperative radiotherapy.
Most of the women were Caucasian, and their racial characteristics were similar. The characteristics of the two groups are shown in Table 1. Although there were more individuals in the group that was not submitted to RT (60% of the sample population), the groups had similar features (age, smoking habit, BMI [body mass index] status, expansion volume, and expansion amount), thus representing possible confounding in this study.
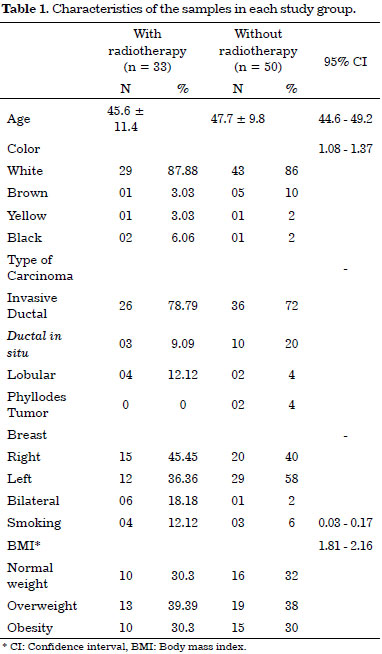
Invasive ductal carcinoma occurred in the majority of patients and accounted for 79% in the RT group and 72% in the non-RT group. In addition, 93.9% of the patients received adjuvant chemotherapy in combination with RT (p = 0.000); the cases were predominantly unilateral with a statistical difference between the groups (p = 0.014).
Few women in both groups smoked, and the number of smokers in both groups was not statistically different, as assessed by Fisher's exact test (p = 0.428). Overall, the BMI was similar between groups. However, we observed a high proportion of overweight and obese women (p = 0.870). According to the boxplot Figure 8, the medians were comparable, and greater variability was observed in the median values in the non-RT group.
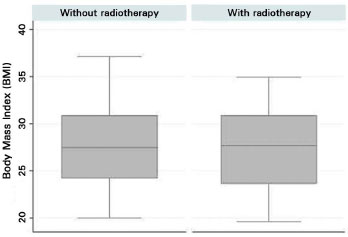
Figure 8. Boxplot of body mass index of the patients who did or did not undergo postmastectomy radiotherapy.
Compared to the women in the non-RT group, more women in the RT group underwent sentinel lymph node biopsy (100% vs. 94%) (p = 0.213). Regarding axillary dissection, statistically significant differences were found in both the groups (p = 0.000); 42% of the women in the RT group were submitted to axillary dissection, while only 6% of the women in the non-RT group underwent this procedure.
The volume of tissue expander (p = 0.932) and expansion (p = 0.992) did not differ between groups. In the RT group, the average volume of expander was 408.1 ml (SD = 74.9) and the average expansion was 391.1 ml (SD = 101.1). In the non-RT group, the average volume of expander was 406.9 ml (SD = 87.4), and the average expansion was 388.0 ml (SD = 125.2) (Figure 9). The average time till the second surgery was 10.8 months (SD = 8.5) for the non-RT group, and 7.9 months (SD = 9.7) for the RT group. There was no statistically significant difference (p = 0.159).
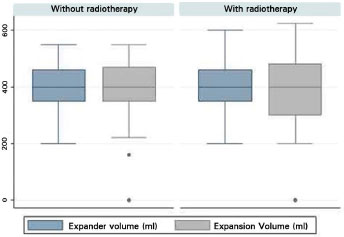
Figure 9 Boxplot of the volume of the expander and expansion between the groups.
Most of the subjects in both the groups (n = 90), most of the individuals who were evaluated did not develop any complications. However, we observed expander displacement (4.8%), emptying (2.4%), infection (2.4%), skin dehiscence (2.4%), and extrusion (1.2%) in the first surgery. In the second surgical procedure, the complications included capsular contracture (7%), extrusion (5.3%), and infection (5.3%). In the first surgery, 18.18% of the patients in the RT group experienced a complication; this reduced to 10.0% in the non-RT group. The risk of these complications was higher in the RT group (p = 0.0001; 95% confidence interval [CI], 1.68-5.4). In the second surgery, the incidence of complications was 46.6% in the RT group. It is important to note that only 68.7% of the individuals in this group were submitted to a second surgery (Table 2).
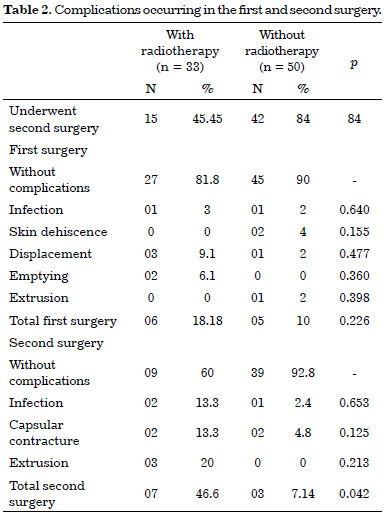
To verify the occurrence of complications in the second surgery and the effect of RT, differences between groups were assessed by Fisher's exact test (p = 0.042); the risk ratio estimated for the occurrence of complications in the second surgery was 3.3 times higher for the RT group (p = 0.000; 95% CI, 1.85-5.9). The use of chemotherapy alone did not show a significant difference in the number of complications in the first (p = 0.166) and second surgeries (p = 0.843). Among patients submitted to axillary dissection and chemotherapy, the risk/complications ratio for the second surgery was 2.28 times higher (p = 0.023; 95% CI, 1.17-4.46), and the risk/complications ratio was 2.8 times higher for RT patients (p = 0.0039; 95% CI, 1.39-5.7). However, the sample included a very high number of individuals submitted to axillary dissection and RT. Therefore, these data should be compared with samples that are relevant to the risks and measures of prevention adopted in this group of patients.
The combination of RT and chemotherapy showed no difference regarding the risk of complications; among patients receiving chemotherapy, the frequency of individuals who also received RT was 93.9%. The risk ratio of receiving both treatments was 0.78 (p = 0.025; 95% CI, 0.65-0.95). Women aged 45 years or older are considered a risk group for complications. However, we did not detect any difference in the sample studied. In an analysis of only those who developed complications, 48.5% of the RT group were older than 45, compared to 52% of the non-RT group (p = 0.754).
The risk analyses suggests that patients exposed to RT and chemotherapy have a higher chance of developing complications in the second surgery. Figures 10-21 illustrate some of the results obtained with the reconstruction.
Figure 10. Postoperative period of 8 months after reconstruction with the expander. Full expansion with 400 ml.
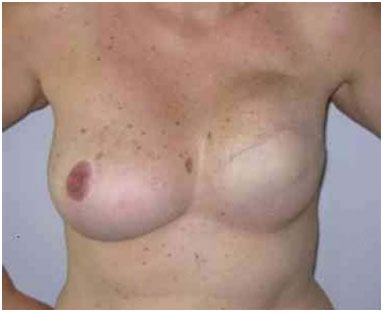
Figure 11. Postoperative period of 1 year after replacement of the expander by the implant in the inframammary fold and symmetrization of the right breast.
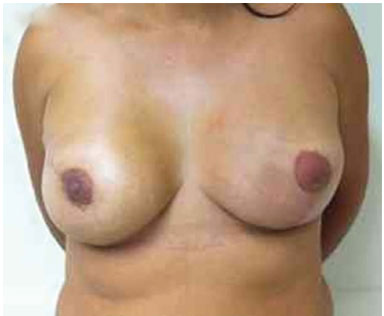
Figure 12. Postoperative period of 2 years after replacement of the expander by the implant in the right breast and symmetrization of the left breast.
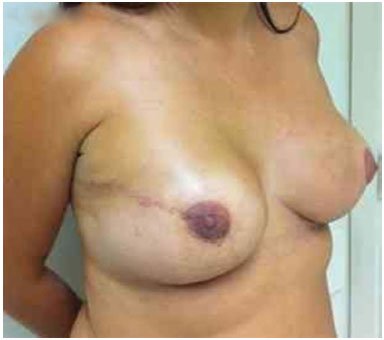
Figure 13. Postoperative period of 1 year after replacement of the expander by the implant in the right breast and symmetrization of the left breast.
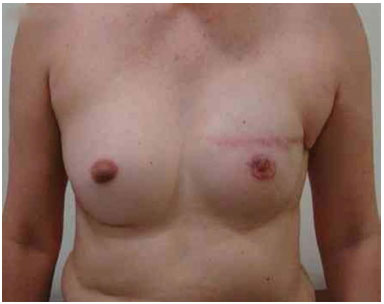
Figure 14. Postoperative period of 1 year after replacement of the expander by the implant in the left breast and symmetrization of the left breast with the prosthesis.
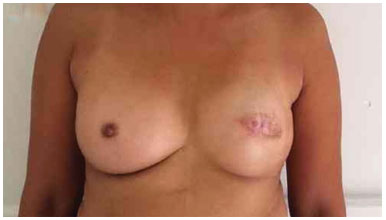
Figure 15. Postoperative period of 3 years after replacement of the expander by the implant in the left breast and symmetrization of the right breast with the prosthesis.
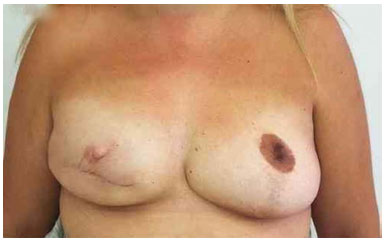
Figure 16. Postoperative period of 5 years after replacement of the expander by the implant in the right breast and symmetrization of the left breast.
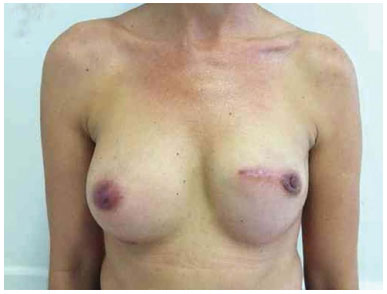
Figure 17. Postoperative period of 8 months after replacement of the expander by the implant in the left breast and symmetrization of the right breast with the prosthesis.
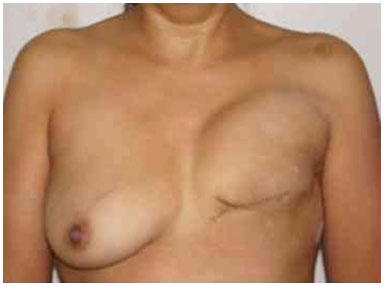
Figure 18. Postoperative period of 6 months after reconstruction with the expander developing upward displacement.
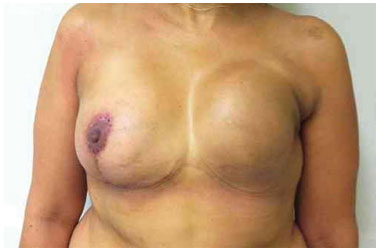
Figure 19. Postoperative period of 3 months after replacement of the expander by the implant in the left breast, repositioning of the inframammary fold, and symmetrization of the breast.
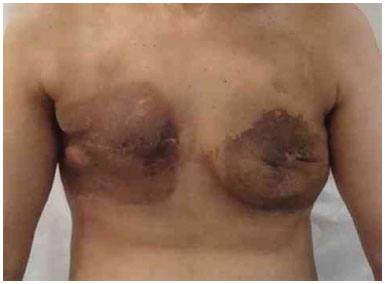
Figure 20. Postoperative period of 6 months after reconstruction with bilateral expander with secondary infection due to radio dermatitis.
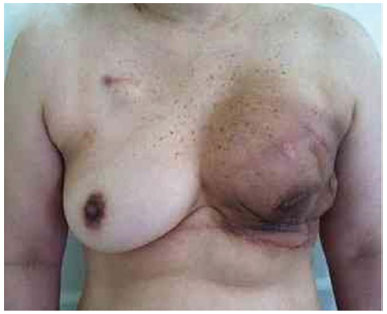
Figure 21. Postoperative period of 4 months after reconstruction with left expander with infection in a patient who underwent radiotherapy.
DISCUSSION
The choice of the most suitable technique for breast reconstruction depends on the type of mastectomy performed, treatment adjuvants, and patient characteristics such as donor area, concomitant systemic disease, and patient preference. In our initial assessment, we presented all methods of breast reconstruction and performed a physical examination to determine the suitability of the donor sites. We then made a joint decision with the patient.
The indications for postmastectomy RT continue to increase. A growing number of women submitted to mastectomy will receive subsequent RT.
Until now, RT has been recommended only for patients with compromised tumor margins, T3 tumors, or > 4 positive lymph nodes. However, several clinical trials have documented the survival advantage of adjuvant RT for patients with stage II tumors, or with less than four lymph nodes involved. However, an increasing number of women undergoing immediate breast reconstruction with positioning of a tissue expander receive irradiation7.
Reports in the literature question the interference of adjuvant treatments such as RT on breast reconstruction, regardless of the type of reconstruction. Additionally, the incidence of complications after autologous reconstruction and irradiation is also quite significant. At the M. D. Anderson Cancer Center, of 32 irradiated patients with transverse rectus abdominis muscle flaps, 87% developed late complications and 28% required an additional flap to repair the breast contour8.
Our study did not evaluate the results of patients who received irradiation before mastectomy, or reflect the experience with irradiated patients during the expansion process. We completed the tissue expansion before starting RT. The expander was replaced by the implant at least 6 months after the completion of RT. Waiting until complete recovery from the effects of RT can significantly reduce the failures associated with breast reconstruction9. Our results are consistent with those showing a negative influence of irradiation on the complications of breast reconstruction with expanders. We observed a complication prevalence of 13.2% in the first surgery, and 17.6% in the second. Four women in the RT group (18.18%) and five (10.0%) in the non-RT group developed complications in the first surgery. Even though there were more subjects in the non-RT group, the prevalence of complications was similar in both groups. In the second surgery, the risk of complications was 3.3 times higher in the RT group (p = 0.000; 95% CI, 1.85-5.9). We need to be aware that this probability can be attributed to an insufficient sample size, and therefore the findings cannot be generalized.
In the second surgery, 7 women in the RT group developed complications, and 3 patients required a rescue surgery from the reconstruction, 2 from an infection, and 1 from an extrusion (2 with a latissimus dorsi and 1 with a transverse abdominal muscle flap). In 2 cases of extrusion, the implant was replaced by a smaller prosthesis. The non-RT group had only 1 case of infection due to careless drain management and required implant removal and subsequent replacement with an expander implant.
Of 83 patients who underwent the first surgery, 57 were submitted to the second surgery and 26 did not finalize the procedure for the following reasons: 20 were still in adjuvant treatment, 3 lost their health insurance, 1 died, and 2 developed complications during the first surgery (radio dermatitis, with secondary infection). Our results are consistent with those reported in previous studies. Almost all studies have shown an increase in the complication rate in patients with a previous history of radiation when compared to non-irradiated patients after reconstruction with breast tissue expansion10-14.
Krueger et al.15, in a study of 81 patients, reported a complication prevalence of 68% among irradiated patients and 31% among non-irradiated patients. Cordeiro et al.16 reported a complication prevalence of 29.7% in the irradiated group and 15.5% in the non-irradiated group. These researchers showed that while irradiated patients had higher rates of complications, the general satisfaction of the patient remained the same in both the irradiated and non-irradiated groups15-17.
Several studies have shown that the use of acellular dermis is a useful adjuvant therapy to minimize complications for immediate breast reconstruction; however, we have no access to bioprosthetic materials because of the high cost. A study published by Spear et al.18 reported an overall complication rate of 12% after expansion with acellular dermis, while the rate of complications after an implant exchange was 2.2%.
CONCLUSION
Patients subjected to RT after reconstruction showed the highest rates of complications. The risk ratio estimated for the occurrence of complications in the second surgery was 3.3 times higher for the RT group.
REFERENCES
1. Brasil. Instituto Nacional Câncer. Estimativa 2012, Rio de Janeiro [Acesso em: 22 Jul. 2013]. Disponível em: http://www2.inca.gov.br/wps/wcm/connect/tiposdecancer/site/home/mama
2. Veronesi U, Saccozzi R, Del Vecchio M, Banfi A, Clemente C, De Lena M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305(1):6-11. PMID: 7015141 DOI: http://dx.doi.org/10.1056/NEJM198107023050102
3. Fisher B, Anderson S. Conservative surgery for the management of invasive and noninvasive carcinoma of the breast: NSABP trials. National Surgical Adjuvant Breast and Bowel Project. World J Surg. 1994;18(1):63-9. DOI: http://dx.doi.org/10.1007/BF00348193
4. Al-Ghazal SK, Sully L, Fallowfield L, Blamey RW. The psychological impact of immediate rather than delayed breast reconstruction. Eur J Surg Oncol. 2000;26(1):17-9. DOI: http://dx.doi.org/10.1053/ejso.1999.0733
5. Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982;69(2):195-208. PMID: 7054790 DOI: http://dx.doi.org/10.1097/00006534-198202000-00001
6. Di Benedetto G, Aquinati A, Santoli M, Bertani A. Which is the best position for the remote injection dome using the adjustable expander/prosthesis in breast reconstruction? A comparative study. Plast Reconstr Surg. 2004;113(6):1629-33. PMID: 15114122 DOI: http://dx.doi.org/10.1097/01.PRS.0000117193.97440.CC
7. Frassica DA, Zellars R. Radiation oncology: the year in review. Curr Opin Oncol. 2002;14(6):594-9. DOI: http://dx.doi.org/10.1097/00001622-200211000-00002
8. Tran NV, Chang DW, Gupta A, Kroll SS, Robb GL. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108(1):78-82. PMID: 11420508 DOI: http://dx.doi.org/10.1097/00006534-200107000-00013
9. Peled AW, Foster RD, Esserman LJ, Park CC, Hwang ES, Fowble B. Increasing the time to expander-implant exchange after postmastectomy radiation therapy reduces expander-implant failure. Plast Reconstr Surg. 2012;130(3):503-9. PMID: 22929235 DOI: http://dx.doi.org/10.1097/PRS.0b013e31825dbf15
10. Benediktsson K, Perbeck L. Capsular contracture around saline-filled and textured subcutaneously-placed implants in irradiated and non-irradiated breast cancer patients: five years of monitoring of a prospective trial. J Plast Reconstr Aesthet Surg. 2006;59(1):27-34. DOI: http://dx.doi.org/10.1016/j.bjps.2005.08.005
11. Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg. 2006;118(4):825-31. DOI: http://dx.doi.org/10.1097/01.prs.0000232362.82402.e8
12. Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105(3):930-42. PMID: 10724252 DOI: http://dx.doi.org/10.1097/00006534-200003000-00016
13. Spear SL, Newman MK, Bedford MS, Schwartz KA, Cohen M, Schwartz JS. A retrospective analysis of outcomes using three common methods for immediate breast reconstruction. Plast Reconstr Surg. 2008;122(2):340-7. PMID: 18626348 DOI: http://dx.doi.org/10.1097/PRS.0b013e31817d6009
14. Peled AW, Stover AC, Foster RD, McGrath MH, Hwang ES. Long-term reconstructive outcomes after expander-implant breast reconstruction with serious infectious or wound-healing complications. Ann Plast Surg. 2012;68(4):369-73. PMID: 22421481 DOI: http://dx.doi.org/10.1097/SAP.0b013e31823aee67
15. Krueger EA, Wilkins EG, Strawderman M, Cederna P, Goldfarb S, Vicini FA. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49(3):713-21. PMID: 11172953 DOI: http://dx.doi.org/10.1016/S0360-3016(00)01402-4
16. Cordeiro PG, Snell L, Heerdt A, McCarthy C. Immediate tissue expander/implast breast reconstruction after salvage mastectomy for cancer recurrence following lumpectomy/irradiation. Plast Reconstr Surg. 2012;129(2):341-50. PMID: 22286416 DOI: http:// dx.doi.org/10.1097/PRS.0b013e318205f203
17. Cordeiro PG, Pusic AL, Disa JJ, McCormick B, VanZee K. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113(3):877-81. PMID: 15108879 DOI: http://dx.doi.org/10.1097/01.PRS.0000105689.84930.E5
18. Spear SL, Parikh PM, Reisin E, Menon NG. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32(3):418-25. DOI: http://dx.doi.org/10.1007/s00266-008-9128-8
1. Universidade Federal de São Paulo, São Paulo, SP, Brazil
2. Universidade Estadual do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
3. Sociedade Brasileira de Cirurgia Plática, São Paulo, SP, Brazil
4. Instituto Brasileiro de Cirurgias Plástica, São Paulo, SP, Brazil
Institution: Instituto Brasileiro de Cirurgia Plastica, São Paulo, SP, Brazil.
Corresponding author:
Andreia Bufoni Farah
Alameda dos Aicás, 1417, Moema
São Paulo, SP, Brasil Zip code 04086003
E-mail: andreiafarah@gmail.com
Article received: July 29, 2013.
Article accepted: May 26, 2014.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter