

Reviw Article - Year 2015 - Volume 30 -
Options for capsulotomy and capsulectomy in the treatment of capsular contracture: are there clinical treatment alternatives to surgery? A literature review
Opções a capsulotomia e capsulectomia no tratamento da contratura capsular: existem alternativas medicamentosas ao tratamento cirúrgico? Revisão de literatura
ABSTRACT
INTRODUCTION: Capsular contracture is among the main complications of surgeries involving breast implants. The most commonly used classification to assess the degree of contracture is the Baker grading system, which divides contractures into grades I, II, III, and IV. Of these, grade III and IV contractures are considered significant. Although several causes have been postulated, the etiology of capsular contracture remains uncertain. Conventional treatment for contracture is based on a surgical approach, specifically capsulotomy or capsulectomy. These procedures, however, are not exempt from morbidity, and patients may develop complications such as dehiscence, hematoma, seroma, pneumothorax, asymmetry, and contracture recurrence. This study provides a review of alternatives to conventional surgery described in the literature.
METHODS: We researched the PubMed and Cochrane Library databases using the following keywords: "capsular contracture", "capsular contracture treatment", and "capsular contracture breast treatment". We identified 991 articles from which we selected those discussing medication options for contracture treatment other than capsulectomy and capsulotomy.
RESULTS: We identified several studies in which drugs, most commonly zafirlukast, were used to reduce capsular contracture.
CONCLUSION: Among the various reported drugs, zafirlukast exhibited good efficacy and a low rate of complication. Triamcinolone also appears to be a good option, although professional assistance would be needed for drug administration via infiltration. The other drugs described would require further investigation.
Keywords: Contracture; Capsular; Drugs; Treatment.
RESUMO
INTRODUÇÃO: A contratura capsular é uma das principais complicações em cirurgias envolvendo implantes mamários. A classificação mais usada para avaliar o grau de contratura é a de Baker, que a divide em graus I, II, III e IV, sendo as de grau III e IV consideradas significativas. Apesar de existirem diversas teorias, a etiologia da contratura capsular permanece incerta. O tratamento convencional para os casos de contratura é a abordagem cirúrgica com realização de capsulotomia ou capsulectomia. Estes procedimentos, no entanto, não estão isentos de morbidades, com complicações como deiscências, hematomas, seromas, pneumotórax, assimetrias e recidiva da contratura. Este estudo faz uma revisão sobre as alternativas ao tratamento cirúrgico convencional, descritas na literatura.
MÉTODOS: Foi realizada pesquisa nas bibliotecas da Pubmed e da Cochrane, utilizando-se os termos: 'capsular contracture', 'capsular contracture treatment' e 'capsular contracture breast treatment'. Foram identificados 991 artigos e selecionados os que discutiam opções medicamentosas para o tratamento de contratura, diferentes de capsulectomia e capsulotomia.
RESULTADOS: Foram encontrados vårios estudos utilizando drogas com o fim de reduzir a contratura capsular, das quais o Zafirlucaste é apresentado em maior número de trabalhos.
CONCLUSÃO: Dentre as várias drogas utilizadas, o Zafirlucaste apresentou boa eficácia, com baixos índices de complicação; a Triancinolona parece ser também uma boa opção, no entanto precisa de profissional habilitado para realizar as infiltrações. As demais drogas necessitam de maiores estudos.
Palavras-chave: Contratura; Capsular; Drogas; Tratamento.
Capsular contracture is a major complication of surgeries involving breast implants. The excessive formation of a fibrous capsule around the implant leads to a spectrum of symptoms ranging from breast hardening and pain to total distortion of the breast shape and volume1. The classification most commonly used to assess the degree of contracture is the Baker Grading system2, which divides cases into grades I, II, III, and IV; of these, grades III and IV are considered significant. In studies published by Mentor and Allergan in 2007 for approval of their implants for use in the United States, the reported incidence rates of contracture in breast augmentation surgeries were 8.1-18.9% and 13.2-17%, respectively. For reconstruction surgeries, the corresponding rates were reported as 8.3-16.3% and 6.7-14.1%, respectively3-6.
Several measures have been proposed to decrease the incidence of contracture, with varying levels of success, including meticulous hemostasis of the implant area, use of preoperative antimicrobial treatment, irrigation of the area and/or implant with antimicrobial solutions, use of textured implants, and selection of the subpectoral plane.
Although several causes have been postulated, the etiology of capsular contracture remains uncertain. In recent years, several factors have been implicated, including hypertrophic scarring, silicone gel leakage, hematoma development, and subclinical infection on the implant surface. Of these, infection is the most prevalent, with the largest amount of accumulated data in its favor7. From a more global perspective, we might consider capsular contracture to result from a series of factors influencing the inflammatory response around the implant. Such a model would agree with a theory of infection, according to which a bacterial biofilm would perpetuate an inflammatory response, and could also include other associated factors that might exacerbate the inflammatory response, such as silicone gel leakage, tissue damage resulting from the surgical technique, and the intensity and quality of the patient's healing process.
Conventional treatment for cases of contracture is based on a surgical approach that incorporates capsulotomy, for which recurrence rates range from 54% to 58%8, or capsulectomy, with a resolution rate of 79%9. These procedures, however, are not exempt from morbidities, and affected patients may develop complications such as dehiscence, hematoma, seroma, pneumothorax, asymmetry, and contracture recurrence. The present study provides a review of treatments described in the literature as alternatives to conventional surgery.
METHODS
We researched the PubMed and Cochrane Library databases using the following keywords: "capsular contracture", "capsular contracture treatment", and "capsular contracture breast treatment". We identified 991 articles, from which we selected those that discussed medication options for contracture treatment other than capsulectomy and capsulotomy.
RESULTS
Twenty-five articles evaluated the use of drugs such as zafirlukast, enalapril, colchicine, indomethacin, triamcinolone, diclofenac sodium topical, and pirfenidone as alternatives to surgical treatment.
The evaluated drugs exhibited significant success rates with low complications. Among these drugs, the efficacy of zafirlukast was evaluated most frequently and reported in the highest number of studies (12 studies). The other drugs have been sporadically described in the literature.
Zafirlukast (Accolate®)
The highest number of studies involved Zafirlukast and yielded converging results. The 12 studies evaluated herein were divided into prospective and clinical trials and histological evaluations of the effects and mechanistic pathophysiology of zafirlukast in contractures. Three of these studies were conducted using animal models.
Despite its original use as an asthma treatment, given its anti-inflammatory properties, Zafirlukast effectively decreases contractures and inhibits leukotrienes (LTC4, LTD4, and LTE4) and myofibroblasts. The effects of this drug, which was approved by the FDA in 1996, on capsular contracture were evaluated by Schlesinger10.
Clinical trials conducted in rats demonstrated positive results with Zafirlukast in terms of decreased capsular thickness, as well as reductions in the vascular density and numbers of mast cells and eosinophils11-13.
Additionally, prospective studies conducted in humans to evaluate the efficacy of zafirlukast in patients with contracture reported similar results after 6 months.
In a study of women with contractures in which the degree of comprise was between 1.5 and 2.0 in 95% of cases and 2.5% of cases received grade III classification, Zafirlukast use improved capsular contracture, with a reduction rate of 73% to grade I according to the Baker grading system and no breast remaining at a grade III level at the end of 6 months. That study also reported a total plus partial Zafirlukast response rate of 75.7%14, similar to that obtained with capsulectomy (i.e., 79% resolution)9. Another prospective study demonstrated improvements in breast contracture in patients treated with Zafirlukast. Prior to administration (D0), patients exhibited an average contracture of 55.89% (equivalent to Baker Grade III); after 6 months (D6)15, this rate decreased to 42.76% (equivalent to Baker Grade II).
A prospective study of 60 patients (120 breasts) with contractures ranging from mild to severe reported results of improved compliance in 22.52% after 6 months of Zafirlukast treatment. However, that study also reported that the effect of Zafirlukast was restricted to the usage period and that a gradual loss of efficacy (i.e., 5.47%) was observed after 1 year16.
A larger clinical trial evaluated 120 women (216 breasts) divided in 2 groups: Group A, patients who used Zafirlukast for 6 months, and Group B, those who used vitamin E. The results demonstrated a cumulative improvement in breast consistency of 24.01% in Group A after 6 months of Zafirlukast treatment. No improvement was observed in Group B, suggesting the particular efficacy of Zafirlukast for the treatment of contracture17.
The side effects of the drug, according to the literature, include headache (12.9%) and nausea (3.1%)15. Sixty-six patients developed hepatitis or liver failure when using normal drug doses, and 2 required a transplant15.
Enalapril
This aldosterone converting enzyme inhibitor (i-ACE) is commonly used to treat hypertension. Various animal model studies have demonstrated the ability of enalapril to inhibit renal and liver fibrosis as a result of its ability to decrease the levels of inflammatory agonists, particularly transforming growth factor (TGF)- β118-23. A clinical trial in mice evaluated the efficacy of enalapril for reducing capsular contracture associated with both textured and smooth implants by measuring capsule thickness after three months of treatment. The results of this trial are shown in Table 1.
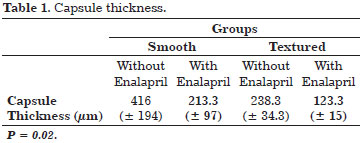
The study results favored the use enalapril in all evaluated parameters.
Flector Tissugel® (Diclofenac)
Based on the anti-inflammatory properties of diclofenac, an adhesive patch containing diclofenac-epolamine has been presented as an alternative to surgical treatment; this patch features the advantages of topical application and fewer systemic side effects, such as gastrointestinal bleeding, in addition to the higher bioavailability of the drug in the desired locations24.
A study of 19 patients (26 breasts) was conducted, using patches containing 180 mg of diclofenac that completely surrounded the breasts with contractures. The effectiveness of this drug was 84.2% in patients presenting grade II-III contractures according to the Baker grading system, with complete resolution. Of the 15.8% of patients who did not respond to treatment, all presented with grade III or IV contractures and had been diagnosed at least 10 months earlier.
Cyclosporine
Cyclosporine is a macrolide obtained from fungi, the main action of which is the suppression of T Helper Cells. This agent also reduces the release of interleukin-2 and consequently reduces inflammatory responses25. In an animal model study conducted to assess the effectiveness of capsular contracture, subjects were divided into 3 groups according to the solution administered intraperitoneally for 30 days: Group I, 1.35 mg/0.1 mL cyclosporine solution; Group II, 0.1 mL saline; and Group III, 0.1 mL activated human cytokine. Mice in these groups were evaluated to determine the capsule thickness, number of pericapsular lymphocytes, collagen maturation, and perivascular capsule infiltration. The capsule thickness results are presented in Table 2.
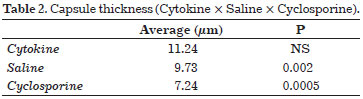
When lymphocytic infiltrates were evaluated, the results were 2.19 for the saline group vs. 1.13 for the cyclosporine group (P = 0.0001). Regarding the perivascular halo, which was graded according to the number of monocytes surrounding pericapsular vessels, we obtained values of 1.22 for saline vs. 0.15 for cyclosporine, respectively (P = 0.0001). No studies in humans have reported corresponding results.
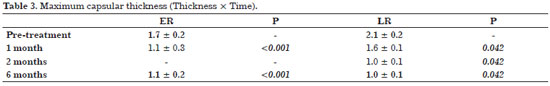
Ultrasound-guided Triamcinolone infiltration
Triamcinolone is a long-acting corticosteroid available in different forms (e.g., oral, injectable, and topical). In a prospective study, intracapsular infiltration was used to treat 25 patients with Baker Grade IV contracture following breast reconstruction or augmentation mammoplasty with silicone implants. In addition to the degree of contracture, the level of patient-reported pain was evaluated using a 10-cm scale ranging from 0 (without pain) to 10 (excruciating pain).
Infiltration was performed aseptically, and patients did not receive other types of medication during the post-operative period. For patients who failed to respond after 30 days, a second infiltration was administered according to the same procedure, and patients were reassessed after an additional 30 days.
Patients receiving a single infiltration were classified as Early Responders (ER), and those receiving a second infiltration as Late Responders (LR). No perforation accidents occurred during the study.
Patients were evaluated 6 months after the first administration of Triamcinolone, and the results are presented in Table 3.
Regarding the patients' complaints of pain, a significant improvement was observed in pain symptoms, as illustrated in Figure 1.

Figure 1. Evaluation of the degree of pain.
DISCUSSION
When faced with capsular contractures following aesthetic and reconstructive surgeries using breast implants, the provision of non-surgical and effective procedures could represent a good medical practice, in addition to alerting patients about possible side effects and potential unsatisfactory results.
Of all the drugs used to treat contractures in the literature, Zafirlukast was most frequently prescribed by surgeons and was found to be safe and able to provide effective, long-lasting results.
Other drugs, including enalapril and cyclosporine, appeared be efficacious for the treatment of capsular contracture in animal studies; however, no studies in humans have been reported to date.
Topical diclofenac administration was more efficacious than capsulectomy for the treatment of contractures. However, these results require further evaluation, possibly through studies featuring better methodologies and higher numbers of patients.
Intralesional triamcinolone administration, which reduced thickness and pain symptoms, appeared to be a good alternative to surgical treatment. However, its administration has been restricted consequent to a higher risk of accidents and requires assistance from an experienced professional.
Our literature search also identified other options, such as pifernidone26, oral corticosteroid27, and external massage with ultrasound28, associated with good levels of resolution, although further studies will be require to confirm those results.
CONCLUSION
Among the drugs reported for the treatment of contracture, Zafirlukast yielded the most positive results, with the greatest number of studies proving its effectiveness. However, in some cases, contracture recurrence may be observed, particularly following drug withdrawal.
Intralesional triamcinolone was also considered an alternative treatment option, although professionals must be available to perform the invasive procedure required for its administration. Other reported drugs require further evaluations and human studies. In conclusion, medication options can be used safely and yield good success rates.
REFERENCES
1. Vinnik CA. Spherical contracture of fibrous capsules around breast implants. Prevention and treatment. Plast Reconstr Surg. 1976;58(5):555-60. http://dx.doi.org/10.1097/00006534-197611000-00004. PMid:981400.
2. Baker J. Augmentation mammoplasty. In: Symposium on Breast Surgery of the Breast; 1978. St. Louis: Mosby; 1978. p. 256-63.
3. U.S. Food and Drug Administration. Summary of safety and effectiveness data. Maryland [cited 2007 Oct 19]. Available from: www.accessdata.fda.gov/cdrh_docs/pdf3/p030053b.pdf.
4. U.S. Food and Drug Administration. Summary of safety effectiveness data. Maryland [cited 2007 Oct 19]. Available from: www.accessdata.fda.gov/cdrh_docs/pdf2/p020056b.pdf.
5. Spear SL, Murphy DK, Slicton A, Walker PS. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):8S-16S. http://dx.doi.org/10.1097/01.prs.0000286580.93214.df. PMid:18090808.
6. Cunningham B. The Mentor core study on silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120(7 Suppl 1):19S-29S. http://dx.doi.org/10.1097/01.prs.0000286574.88752.04. PMid:18090810.
7. Adams WP JR. Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg. 2009;36(1):119-26, vii. http://dx.doi.org/10.1016/j.cps.2008.08.007. PMid:19055967.
8. Moufarrege R, Beauregard G, Bosse JP, Papillon J, Perras C. Outcome of mammary capsulotomies. Ann Plast Surg. 1987;19(1):62-4. http://dx.doi.org/10.1097/00000637-198707000-00010. PMid:3631862.
9. Embrey M, Adams EE, Cunningham B, Peters W, Young VL, Carlo GL. A review of the literature on the etiology of capsular contracture and a pilot study to determine the outcome of capsular contracture interventions. Aesthetic Plast Surg. 1999;23(3):197-206. http://dx.doi.org/10.1007/s002669900268. PMid:10384019.
10. Schlesinger SL, Ellenbogen R, Desvigne MN, Svehlak S, Heck R. Zafirlukast (Accolate): A new treatment for capsular contracture. Aesthet Surg J. 2002;22(4):329-36. http://dx.doi.org/10.1067/maj.2002.126753. PMid:19331987.
11. Zimman OA, Toblli J, Stella I, Ferder M, Ferder L, Inserra F. The effects of angiotensin-converting-enzyme inhibitors on the fibrous envelope around mammary implants. Plast Reconstr Surg. 2007;120(7):2025-33.
12. Bastos EM, Sabino Neto M, Garcia EB, Veiga DF, Han YA, Denadai R, et al. Effect of zafirlukast on capsular contracture around silicone implants in rats. Acta Cir Bras. 2012;27(1):1-6. http://dx.doi.org/10.1590/S0102-86502012000100001. PMid:22159431.
13. Spano A, Palmieri B, Taidelli TP, Nava MB. Reduction of capsular thickness around silicone breast implants by zafirlukast in rats. Eur Surg Res. 2008;41(1):8-14. http://dx.doi.org/10.1159/000121501. PMid:18367842.
14. Reid RR, Greve SD, Casas LA. The effect of zafirlukast (Accolate) on early capsular contracture in the primary augmentation patient: a pilot study. Aesthet Surg J. 2005;25(1):26-30. http://dx.doi.org/10.1016/j.asj.2004.12.003. PMid:19338783.
15. Scuderi N, Mazzocchi M, Fioramonti P, Bistoni G. The effects of zafirlukast on capsular contracture: preliminary report. Aesthetic Plast Surg. 2006;30(5):513-20. http://dx.doi.org/10.1007/s00266-006-0038-3. PMid:16977359.
16. Scuderi N, Mazzocchi M, Rubino C. Effects of zafirlukast on capsular contracture: controlled study measuring the mammary compliance. Int J Immunopathol Pharmacol. 2007;20(3):577-84. PMid:17880770.
17. Mazzocchi M, Dessy LA, Alfano C, Scuderi N. Effects of zafirlukast on capsular contracture: long-term results. Int J Immunopathol Pharmacol. 2012;25(4):935-44. PMid:23298484.
18. Ferder L, Inserra F, Romano L, Ercole L, Pszenny V. Decreased glomerulosclerosis in aging by angiotensin-converting enzyme inhibitors. J Am Soc Nephrol. 1994;5(4):1147-52. PMid:7849256.
19. Inserra F, Romano LA, de Cavanagh EM, Ercole L, Ferder LF, Gomez RA. Renal interstitial sclerosis in aging: effects of enalapril and nifedipine. J Am Soc Nephrol. 1996;7(5):676-80. PMid:8738801.
20. Ferder LF, Inserra F, Basso N. Advances in our understanding of aging: role of the renin-angiotensin system. Curr Opin Pharmacol. 2002;2(2):189-94. http://dx.doi.org/10.1016/S1471-4892(02)00139-X. PMid:11950632.
21. Ferder LF, Inserra F, Basso N. Effects of renin-angiotensin system blockade in the aging kidney. Exp Gerontol. 2003;38(3):237-44. http://dx.doi.org/10.1016/S0531-5565(02)00264-4. PMid:12581787.
22. Cavanagh EM, Inserra F, Toblli J, Stella I, Fraga CG, Ferder L. Enalapril attenuates oxidative stress in diabetic rats. Hypertension. 2001;38(5):1130-6. http://dx.doi.org/10.1161/hy1101.092845. PMid:11711510.
23. Toblli JE, Ferder L, Stella I, Angerosa M, Inserra F. Enalapril prevents fatty liver in nephrotic rats. J Nephrol. 2002;15(4):358-67. PMid:12243364.
24. Le Louarn C, Buis J, Auclair E. Flector tissugel used to treat capsular contracture after breast augmentation surgery. Aesthetic Plast Surg. 2008;32(3):453-8. http://dx.doi.org/10.1007/s00266-008-9123-0. PMid:18389304.
25. Miller AS, Tarpley SK, Willard VV, Reynolds GD. Alteration of fibrous formation by use of immunomodulation capsule. Aesth Plast J. 1998;18(5):346-52.
26. Gancedo M, Ruiz-Corro L, Salazar-Montes A, Rincón AR, Armendáriz-Borunda J. Pirfenidone prevents capsular contracture after mammary implantation. Aesthetic Plast Surg. 2008;32(1):32-40. http://dx.doi.org/10.1007/s00266-007-9051-4. PMid:17968613.
27. Lemperle G, Exner K. Effect of cortisone on capsular contracture in double-lumen breast implants: ten years' experience. Aesthetic Plast Surg. 1993;17(4):317-23. http://dx.doi.org/10.1007/BF00437105. PMid:8273534.
28. Planas J, Migliano E, Wagenfuhr J JR, Castillo S. External ultrasonic treatment of capsular contractures in breast implants. Aesthetic Plast Surg. 1997;21(6):395-7. http://dx.doi.org/10.1007/s002669900143. PMid:9354599.
1. Departamento de Comissão de Feridas, Instituto Medicina Integral Fernando Figueira (IMIP), Recife, PE, Brazil
2. Universidade de São Paulo (USP), São Paulo, SP, Brazil
3. Universidade Federal de Pernambuco (UFPE), Recife, PE, Brazil
Institution: Study carried out at Instituto de Medicina Integral Professor Fernando Figueira - IMIP, Recife, PE, Brazil.
Corresponding author:
Kleiton Cardozo de Oliveira
Instituto Medicina Integral Fernando Figueira
Av. Brasil, 580, Casa 311 - Bairro Universitário, 112
Caruaru, SP, Brazil Zip Code 55016-360
E-mail: kleitoncardozo@hotmail.com
Article received: December 6, 2013.
Article accepted: February 20, 2014.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter