

Original Article - Year 2014 - Volume 29 -
Reconstruction of chest wall after resection of large tumors
Reconstrução da parede torácica após a ressecção de extensos tumores
ABSTRACT
INTRODUCTION: Complex procedures and implants are required in the final stages of chest wall reconstruction after tumor excision. This process requires multidisciplinary care with participation from thoracic and plastic surgeons, a radiologist, and a physical therapist. The goal of this study was to describe the options for chest wall reconstruction after neoplasm resection at Hospital Sarah Brasilia.
METHOD: A retrospective study of one-time chest wall reconstruction after tumor excision, respiratory physical therapy with noninvasive ventilation, and exercises was conducted.
RESULTS: Between 2007 and 2012, 10 patients underwent surgery (seven men, three women; age range: 10-31 years); eight patients had metastatic thoracic tumors (e.g., osteosarcoma, synovial sarcoma, sclerosing epithelioid fibrosarcoma, and rhabdomyosarcoma) and two had tumors originating from the chest wall (fibromatosis and chondrosarcoma). The outcomes were good after the immediate postoperative period, with extubation occurring at the end of surgery and chest tube removal between the fifth and eighth postoperative day. Three cases (30%) involved complications of atelectasis (10%), tumor recurrence (10%), or death.
CONCLUSION: One-time chest wall reconstruction using polypropylene mesh, polymethylmethacrylate, and muscle flaps was possible and was associated with early recovery of pulmonary function and a low rate of immediate complications.
Keywords: Chest wall reconstruction; Thoracic tumor; Polymethylmethacrylate.
RESUMO
INTRODUÇÃO: Nos estágios finais da reconstrução torácica, consequente a exéreses tumorais, são necessários procedimentos complexos e implantes. O que requer cuidados multidisciplinares, com a participação dos cirurgiões torácicos, plástico, radiologista e fisioterapeuta. O objetivo foi descrever as opções de reconstrução torácica após ressecção de neoplasia, realizado no Hospital Sarah Brasília.
MÉTODO: Estudo retrospectivo de reconstrução torácica em tempo único, após excisão de tumor, fisioterapia respiratória com ventilação não invasiva e exercícios.
RESULTADOS: Entre 2007 a 2012 foram operados 10 pacientes, sete homens e três mulheres; idade 10 a 31 anos; oito apresentavam tumores torácicos metastáticos (osteosarcoma, sinoviosarcoma, Fibrosarcoma epitelioide esclerosante e Rabdomiosarcoma) e dois originários da parede torácica (fibromatose e condrosarcoma). Observou-se boa evolução no pós-operatório imediato, com extubação ao final da cirurgia, retirada do dreno torácico entre 5° e 8° PO. As complicações foram: atelectasia (10%), recorrência tumoral (10%), e óbito em 3 (30%) casos .
CONCLUSÃO: Foi possível a reconstrução torácica em tempo único utilizando tela de polipropileno, polimetilmetacrilato e retalhos musculares, com recuperação precoce da função pulmonar e baixo índice de complicações imediatas.
Palavras-chave: Reconstrução torácica; Tumor torácico; Polimetilmetacrilato.
The concept of thoracoplasty was first described in 1890 for the treatment of chronic empyema and remains in use in the 21st century. It is considered by many authors to be a mutilating, low-tolerance procedure and is usually reserved as a last resort for the treatment of malignant neoplasms1-4. The concept of thoracoplasty involves the removal of all costal arches for the treatment of chest infections. The use of muscle flaps for chest wall reconstruction has been described since 19705-,7. Muscles used for this procedure include the latissimus dorsi, serratus anterior, pectoralis major, and rectus abdominis. Use of the greater omentum has been described in some cases8. There have been many advances in chest wall reconstruction using such flaps throughout the years; however, respiratory complications and death have been reported after major resection without chest wall reconstruction6-8. Use of a rigid prosthesis for chest wall construction can reduce the frequency of complications9. Unfortunately, there are limitations associated with obtaining and using autologous materials, and an ideal alloplastic material has yet to be identified. In the 21st century, various autologous materials such as the fascia lata, dura mater, cartilage, and pericardium have been used in reconstructive procedures. Furthermore, biomaterials such as titanium, carbon fiber, acrylic (PMMA), nylon, teflon, silicone, and polypropylene (Marlex R) have been employed as options in situations involving the limited use of autologous materials or large defects, to reduce donor site morbidity when performing reconstruction in patients with more advanced ages, for graft resorption, and in cases involving a loss of the physical properties of bones10,11. The ideal synthetic material should be inert, biocompatible, nonconductive, resistant to infection, nonmagnetic, lightweight, rigid, and easy to generate and apply.
OBJECTIVE
The objective of this research was to conduct a retrospective study of the results from 10 cases subjected to chest wall reconstruction and rehabilitation following surgical resection of chest wall tumors under the care of a multidisciplinary team at the Hospital Sarah Brasilia.
METHOD
A retrospective study of the medical records of patients subjected to chest wall tumor removal, reconstruction, and postoperative physical therapy at the Hospital Sarah Brasília between 2007 and 2012 was conducted. The following data were studied: age; sex; tumor origin, location, and size; extent of the excision; type of chest wall reconstruction; postoperative outcome; and immediate- and late-onset complications. The research was assessed and approved by the CEP/APS (Research Ethics Committee).
Preoperative
Laboratory tests, spirometry (Table 1) and imaging tests.
Surgical Technique
Under general anesthesia, a thoracotomy was performed along with removal of the enlarged tumor through a skin incision; this procedure included the previous chest wall tumor biopsy site and previously irradiated tissue. The compromised ribs and margins up to 3cm, tumor within the chest cavity, and involved structures involved such as the pleura, lung, hilum, or diaphragm were removed. Tumor excision and reconstruction were performed as a single procedure in all cases. Chest wall reconstructions were semirigid with polypropylene mesh or rigid with polymethylmethacrylate in the form of bone cement according to the "Sandwich" technique; this was molded during surgery and closed with the surrounding muscle flaps. At least two edges of the prosthesis were fixed to the marginal rigid structure through tension distribution with a 0 Prolene suture thread. The implant was covered with vascular tissue from the pectoral muscle, rectus abdominis, serratus, or latissimus dorsi by sealing the pleural cavity with posterior chest drainage.
Postoperative
Hospitalization in the first stage occurred at a postoperative recovery center. Prophylactic antibiotics and enoxaparin were administered, and patients were subjected to radiological tests and a postoperative control computed tomography (CT) scan. A patient controlled analgesia (PCA) pump for intravenous (IV) administration of a morphine solution (0.5 mg/ml) at an on-demand dose of 2mg until IV POP was used as needed; otherwise, an epidural PCA with a 0.2% 4 ml/h ropivacaine solution, bolus of 4.0ml, and 30-minute lockout interval was installed.
Postoperative Physical Therapy
In this study, postoperative chest physical therapy mainly aimed to at maintain airways patency and promoting lung expansion. Noninvasive positive-pressure ventilation (NPPV) was used at two pressure levels via intermittent bilevel positive airway pressure (BIPAP) with a nasal or oronasal mask interface at least three times per for one hour per session, and supplemental oxygen was provided if required. Additionally, the following were used as preventive measures for pulmonary complications: physical therapistapplied bronchial hygiene techniques through voluntary or assisted coughing, pulmonary expansion techniques associated with the ventilatory pattern, and exercises for extremity mobilization and early ambulation.
Assessment of Clinical Parameters
The following were assessed to monitor patients under NPPV: respiratory rate, heart rate, use of accessory muscles while breathing, paradoxical breathing, chest expandability, patient comfort, coordination of respiratory movement such as apparatus triggering, mental status, and blood gas levels (oxygen saturation, improvement of hypercapnia, and respiratory acidosis). A sling and elastic chest strap were used.
RESULTS
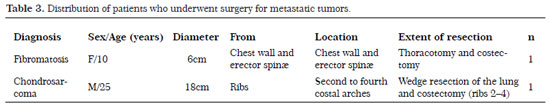
During the five-year study period (2007-2012),10 patients underwent surgery, including seven men and three women with an age range of 10-31 years; eight had metastatic tumors (four osteosarcomas, two synovial sarcomas, one sclerosing epithelioid fibrosarcoma, and one rhabdomyosarcoma) and two had tumors that originated from the chest wall (fibromatosis and chondrosarcoma). The mean follow-up time was 5 years (range, 1-10 years). The tumor types, origins, dimensions, and locations are described in Tables 2 and 3.
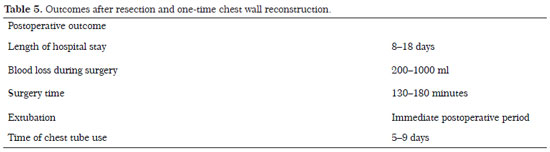
The most frequent site of osteosarcoma origin was the tibia (50% of cases). For the parietal reconstruction procedures, a double layer of polypropylene mesh and a methyl methacrylate mold plate were employed with or without muscle flaps in four cases (Figures 1 and 2), with polypropylene mesh with or without muscle flaps in four cases (Figures 3 and 4), and with muscle reconstruction alone in two cases (Table 4). The outcomes during the postoperative period were good, with extubation at the end of the surgery and spontaneous ventilation with oxygen supplementation at 1 L/min to maintain an oxygen saturation between 97 and 100% with a partial carbon dioxide (pCO2)/partial oxygen pressure (pO2) ratio of 40.9/131; the chest tube was removed between the fifth and eighth POP (Table 4).

Figure 1. Right side thoracotomy during surgery for en bloc lung resection (middle and lower bilobectomy) and partial resection of the diaphragm and ribs 5-11, sequential ligation of veins from the middle and lower lobes and of arterial branches to the middle and lower lobes; sectioning and mechanical suturing of the intermediate bronchus; and parietal reconstruction with a double-layer polypropylene mesh and methyl methacrylate mold plate. The mesh was sutured around the bony edges of the transverse processes and anterior costal cartilages; the diaphragm was sutured and initially reinserted on the thoracoabdominal wall and covered with a myocutaneous flap.

Figure 2. (A) Control X-ray. Note the bone cement on the reconstructed chest wall; (B) Result on the day 30 after surgery; (C) Preoperative magnetic resonance imaging (MRI); (D) Postoperative MRI of the reconstruction with PMMA.

Figure 3. Surgery and postoperative findings in case 2. A bronchoscopy and selective orotracheal intubation were performed under general anesthesia with the patient in the lateral decubitus position. (A) Tumor marking, including the prior biopsy area; (B) Right anterior posterolateral thoracotomy, reflecting the trapezius muscle with sectioning from the latissimus dorsi to the serratus anterior and approach from the cavity in the second intercostal space; (C) Anterior and posterior section from ribs 3-9 of the costal arches, ligature of the intercostal vessels, pneumonectomy, dissection, and mechanical suturing ligation of the pulmonary artery, left bronchus, and the two pulmonary veins. (D) En bloc part removed with the lung and rib cage. The blood volume loss was 1000 ml, and one unit of packed red blood cells was transfused. Reconstruction of the anterolateral chest wall with polypropylene mesh sutured with 0 Prolene thread; approximation of the pectoralis and latissimus dorsi, which were also fixed on the mesh. Closure of subcutaneous tissue and skin; results after 6 months.

Figure 4. Preoperative and postoperative MRI results after thoracotomy and reconstruction with muscle flaps.

Complications included atelectasis (10%) and chest wall tumor recurrence (10%) after 4 years; three (30%) cases succumbed to death (Table 5). Cases involving death occurred between 1 and 2 years after thoracoplasty and were caused by respiratory failure consequent to progression of the metastatic pulmonary disease or kidney failure consequent to chemotherapy. There were no infectious complications or losses of flaps.
DISCUSSION
In this study, the most frequently diagnosed tumor type was a metastatic bone tumor on the chest wall in 80% of the cases12. Among the chest wall tumors presented in this series of cases, chondrosarcoma is the most common primary malignant bone tumor in adults, and Ewing's sarcoma is most common in children13. Fibrous dysplasia is the most prevalent benign tumor of the bony chest wall, accounting for approximately 30% of such tumors13. Traditionally, tumors that exhibit extensive cortical destruction and mass formation in adjacent tissues on imaging are classified as malignant. Bone destruction is best studied using CT, whereas extra bone formation is best studied using MRI; therefore, it is necessary to conduct both CT and MRI examinations. It is not always easy to diagnose a primary malignant tumor from imaging findings; these tumors are best assessed using the mineralization characteristics of the bone matrix pattern12,13. The clinical history (patient age and underlying diseases such as osteosarcomas and other tumors) and imaging findings (e.g., signal intensity features seen on MRI) can assist with a diagnosis of chest wall tumors12.
Chest wall tumor excision must meet the criteria for oncologic surgery, including removal of the tumor with a safety margin; however, this procedure must also provide conditions for rigid to semirigid chest wall reconstruction, thus avoiding complications that arise from chest wall instability. Some authors recommend rigid stabilization of the chest wall for a better postoperative recovery5,7. The cases presented in the present study featured large and complex tumors that involved multiple chest wall structures, and most cases (80%) resulted in metastasis. Patients diagnosed with sarcoma had previously been treated according to the protocols for chemotherapy, radiation therapy, and surgery to achieve local control of the disease1. The main aspects of parietal chest wall reconstruction were skeletal stabilization and soft tissue synthesis10,11. In cases involving minor excisions extending 5-7 cm and the removal of two or three costal arches, those with good muscle coverage, or those involving the resection of tumors located laterally or dorsally, thoracic cage reconstruction was not required2,3. For oncological surgical treatment, tumor removal including the muscles, rib cage, and adjacent structures was required as described in this series of cases as well as in the literature. Resectioning of the rib cage may result in rib segments that can cause skin injuries, paradoxical chest movement, and deformity of the body contours around the chest. Areas involving the removal of large tumors can be resectioned and reconstructed using muscle flaps1; however, intensive care and mechanical ventilation are often required because of the instability generated on the chest wall. Several authors have shown that chest wall stabilization associated with good muscle coverage reduces the hospital stay length and provides adequate postoperative pulmonary function. Chest wall reconstruction with a double-layer polypropylene mesh and methyl methacrylate mold plate has been performed since 19724,5,10,11. Polypropylene mesh is also indicated in some cases involving smaller excisions, as it is permeable, easy to handle, highly resistant, durable, and resistant to infection; however, this mesh can cause severe fibrosis if placed in contact with the lungs, which complicates reoperations.
The polypropylene prosthesis and methyl methacrylate mold plate must be fixed in the rigid bone structure at a minimum of two edges and should be covered with vascularized tissue (muscle or omentum) by sealing the pleural cavity. The muscles used for this purpose include the serratus, pectoralis major, and latissimus dorsi. Luz et al. demonstrated the application of a myocutaneous latissimus dorsi flap for the reconstruction of large chest wall defects9. An alternative to the use of allogeneic material is the thoracolumbar fascia, which is associated with the latissimus dorsi muscle and used in cases involving infection of the chest wall7. It is worth noting that whenever possible, the two top costal arches should be maintained when performing a thoracoplasty if they are not affected by the tumor in order to preserve the contour of the shoulder, as resection of these arches leads to worse aesthetic results. In cases involving infections, the use of methyl methacrylate is not recommended.
The incidence of of immediate postoperative complications was 10%, with one case of atelectasis; that of late-onset complications (within 1-4 years after chest wall reconstruction) was 40%, characterized by tumor recurrence in 10% and death due to the spread of metastatic disease in 30%. Fouad4 et al. described a 45.5% incidence rate of immediate postoperative complications such as infection, hematoma, pneumonia, and respiratory failure and a 40% rate of late-onset complications, including local recurrence in 18% of cases, as well as metastases and infection. It has been observed that survival after chest wall tumor removal depends on the time of diagnosis, histological type of the tumor, possibility of resection with free margins, and presence of metastases. Of the two cases in the present study study with death as an outcome, one resulted from osteoblastic osteosarcoma and the other from tibial osteosarcoma. As the latter case was first diagnosed in 1993 with evolution to chest metastasis 17 years after the initial disease diagnosis and treatment, this death occurred 2 years after the procedure as a result of pulmonary metastatic disease. It is important to emphasize that proper pulmonary functional reserve is the preoperative condition required to perform the thoracoplasty14. This is assessed via spirometry with a FEV1 assessment, and it is estimated that the critical values of a volume >800 ml or 40% of the forecast are sufficient. According to the Guidelines of the Pneumology Society, the FEV1 and FEV1PPO are spirometric variables that must be assessed during the thoracotomy postoperative period (POP) with pneumonectomy14. Surgical procedures such as thoracotomy interfere with pulmonary mechanics and tend to lead to the development of restrictive ventilatory changes and a reduction of the FEV1 and FVC. These procedures may also lead to contralateral pulmonary repercussions in cases where the removal of more than four costal arches is necessary; a two-step procedure is recommended for such cases. For the cases described in this study, up to four costal arches were resectioned, which allowed a single excision and reconstruction procedure.
Pulmonary complications are important causes of morbidity after chest wall surgery and have contributed to significant increases in the costs associated with health care as well as intensive care unit and hospital stays14; however, using a multidisciplinary structure and chest wall reconstruction, it was possible to minimize the immediate complications. Physical therapy interventions have contributed to a reduction in postoperative complications and have been regularly used during both the pre- and postoperative periods associated with chest wall surgeries to prevent clinical and pulmonary complications, as described in this research.
CONCLUSION
One-time chest wall reconstruction was demonstrated as feasible for patients subjected to the excision of large chest wall tumors with or without rigid chest wall reconstruction. Treatment was conducted by a multidisciplinary team, and patients exhibited early recovery of pulmonary function and a low rate of immediate complications.
REFERENCES
1. Banic A, Ris HB, Erni D, Striffeler H. Free latissimus dorsi flaps for chest wall repair after complete resection of infected sternum. Ann Thorac Surg. 1995;60:1028-32.
2. Mansour KA, Thourani VH, Losken A, Reeves JG,Miller JI Jr, Carlson GW, et al. Chest wall resections and reconstruction: A 25-year experience. Ann Thorac Surg. 2002;73:1720-6.
3. Fouad A F. Chest Wall Resection and Reconstruction: Analysis of 11 Cases after Methylmethacrylate Reconstruction. Journal of the Egyptian Nat. Cancer Inst. 2006;18(3):175-182.
4. Miller DL, Force SD, Pickens A, Fernandez FG, Luu T, Mansour KA. Chest Wall Reconstruction Using Biomaterials. Ann Thorac Surg. 2013;95(3):1050-6.
5. Novoa N, Benito P, Jiménez MF, Juan A, Aranda JL, et al. Reconstruction of chest wall defects after resection of large neoplasms:Ten-year experience. Interact CardioVasc Thorac Surg.2005;4:250-55.
6. Raffoul W, Dusmet Ml, Landry M, Ris HB. A Novel Technique for the Reconstruction of Infected Full-Thickness Chest Wall Defects. Ann Thorac Surg . 2001;72:1720-4.
7. Chang RR, Mehrara BJ, Hu QY, Disa JJ, Cordeiro PG. Reconstruction of complex oncologic chest wall defects: A 10-year experience. Ann Plast Surg. 2004,52:471-79.
8. Tavares FM, Menezes CM, Moscozo MV, Xavier GR, Oliveira GM, Amorim JrM, et al. ,Retalho de omento: uma alternativa em cirurgia reparadora da parede torácica .Rev Bras Cir Plást . 2011;26(2)224-32.
9. Luz DP, Lobo CA, Hiraki P, Okada A, Montag E, Ferreira MC. Retalho miocutâneo de latíssimo do dorso em V-Y para reconstrução de grandes defeitos torácicos extensos. Rev Bras Cir Plást. 2010;25(1)52-55.
10. Costa PR, Melo JR, Andrade JrJ, Bezerra MM, Neves LJ, Araújo JM. Reconstrução da Parede Torácica com Metilmetacrilato: Relato de Caso. Rev Bras Cir Plást. 2007;22(4)345-8.
11. Kilic D, Gungor A, Kavukcu S, Okten I, Ozdemir N, Akal M, et al. Comparison of mersilene mesh-methyl metacrylate sandwich and polytetrafluoroethylene grafts for chest wall reconstruction. J Invest Surg. 2006;19(6):353-60.
12. O'Sullivan P, O'Dwyer H, Flint J, Munk PL, Muller NL. Malignant chest wall neoplasms of bone and cartilage: a pictorial review of CT and MR findings.Br J Radiol. 2007;80(956):678-684.
13. Meyer CA, White CS. Cartilaginous disorders of the chest. RadioGraphics 1998;18(5):1109-1123.
14. Reeve JC, Nicol K, Stiller K, McPherson KM, Denehy L. Does physiotherapy reduce the incidence of posoperative complications in patients following pulmonary resection via thoracotomy? A protocol for a randomised controlled trial. J Cardiothoracic Surg. 2008;3(1):48.
15. Pereira CA. Espirometria. J Pneumol. 2002;28(3)587-92.
1. Acting Member of the SBCP (Brazilian Society of Plastic Surgery) - Plastic Surgeon at Hospital Sarah Brasilia
2. Acting Member of the SBCP (Brazilian Society of Plastic Surgery) - Plastic Surgeon at Hospital Sarah Brasilia
3. Acting Member of the SBCT (Brazilian Society of Thoracic Surgery) - Thoracic Surgeon of the Hospital Sarah Network
4. Member of the SBR (Brazilian Society of Radiology) - Radiologist at the Hospital Sarah Network
5. Master of Science in Rehabilitation - Physical Therapist at the Hospital Sarah Network
Institution: Hospital Sarah.
Corresponding author:
Katia Torees Batista
SMHS - Quadra 301 - Bloco A
Brasília, DF, Brazil Zip code: 70335-901
E-mail: katiatb@terra.com.br
Article submitted on: July 30, 2013.
Article accepted on: December 13, 2014.




 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter