

Original Article - Year 2014 - Volume 29 -
Reduction mastoplasty with silicone implants: When is it indicated?
Mastoplastia redutora associada a implante de silicone: quando indico?
ABSTRACT
INTRODUCTION: Patient dissatisfaction with reduction mammoplasty outcomes can occur, especially in cases of ptosis accompanied by excessive flaccidity, striations, and a higher fat than glandular content. In such cases, achieving long-lasting results is very difficult. Small-volume breast implants can be placed during the reduction mammoplasty with the purpose of obtaining better breast shape, contour, and projection as well as greater long-term satisfaction.
METHOD: Between 1997 and 2012, 264 patients aged 27-55 years (mean, 38) underwent reduction mammoplasty with immediate placement of breast implants.
RESULTS: Satisfactory results were obtained, with adequate filling of the upper pole, increased breast firmness, and statistical reduction in postoperative ptosis. Two cases of carcinoma in situ were identified in the pathological exam.
CONCLUSION: Reduction mastoplasty associated with silicone implants is safe for selected cases.
Keywords: Reduction mammoplasty; Breast hypertrophy; Silicone Implants; Carcinoma in situ; Breast ptosis.
RESUMO
Introdução: Insatisfação dos pacientes com resultado de mamoplastia redutora pode ser identificado em alguns casos, especialmente quando apresentam ptose acompanhada de flacidez excessive, estrias, e ainda, componente mamário mais gorduroso que glandular. Nesses tipos de pacientes, é muito difícil conseguir bons resultados por longo período. Implantes mamários de pequeno volume, podem ser colocados no mesmo tempo da mamoplastia redutora com o objetivo de se obter melhor forma, contorno e projeção das mamas, com maior satisfação a longo prazo.
MÉTODO: No período de 1997 a 2012, duzentos e sessenta e quatro pacientes com idade entre 27e 55 anos (idade média de 38), foram submetidas à mamoplastia redutora com imediata colocação de implante mamário.
RESULTADOS: Foram obtidos resultados satisfatórios, com adequado preenchimento do pólo superior, mamas firmes e reduzida estatística de ptose pós-operatória. Foram identificados dois casos de carcinoma in sito, como achados no anátomo-patológico.
CONCLUSÃO: Mastoplastia redutora associada a implantes de silicone é um procedimento seguro para casos selecionados.
Palavras-chave: Mamoplastia redutora; Hipertrofia mamária; Implantes de silicone; Carcinoma in situ; Ptose de mamas.
Aesthetic breast procedures are usually divided into breast augmentation, reduction mammoplasty, and mastopexy. Their main objective is to improve breast shape, symmetry, and size.
Since 1930, breast surgery procedures have preserved the blood supply of the nipple-areola complex (NAC)1. Many techniques and refinements have been developed over the past five decades with the aim of treating different types and degrees of ptosis, hypomastia, and hypertrophy, which has increased the popularity of this procedure accordingly2,3,4.
Since 1997, some patients have sought treatment for breast hypertrophy accompanied by large amounts of flaccid skin and striations by requesting the simultaneous placement of implants. In our experience, some patients with these characteristics who underwent reduction mammoplasty only experienced unsatisfactory long-term results that required revision surgery for the correction of ptosis and the upper pole5,6. It is becoming increasingly common for patients to request implants to obtain better shape and aesthetic results (Figures 1A-1D); however, this combination of procedures has not been published to date.
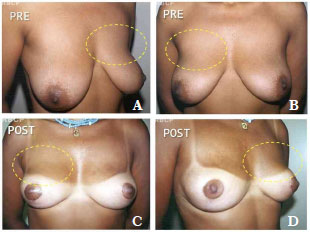
Figure 1. A-D. Pre- and post-operative aspect showing lack of projection of the upper pole in a patient who underwent reduction mammoplasty without breast implant placement (11 months after surgery).
OBJECTIVE
The objective of this study was to report our experience with the simultaneous use of low-volume silicone implants and reduction mammoplasty in 264 patients with breast hypertrophy and excessive skin flaccidity over an observation period > 16 years.
METHOD
Between February 1997 and December 2012, 264 patients (27-55 years; mean age, 28 years) underwent reduction mammoplasty with placement of small-volume silicone implants.
The inclusion criteria were:
1) desire for breast implants and shape correction;
2) flaccid skin, ptosis, and breast hypertrophyTimes New Roman and
3) breasts with a higher fat than glandular content.
The exclusion criteria were:
1) refusal of breast implantsTimes New Roman;
2) young age with a higher breast glandular than fat volume; and
3) severe systemic diseases. Patients who previously underwent bariatric surgery were not excluded.
Surgical Technique
The surgical technique used was based on the technique of Pitanguy . In this procedure, a line is drawn from the clavicular midpoint to the areola to divide the breast into two parts. Lateralization of the NAC is corrected when possible. Point E is marked under the guidance of the projection of the inframammary fold 18-20 cm from the manubrium of the sternum. Points B and C are determined by bi-digital maneuver and 89 cm distant to point A. The distance between points B and C depends on the skin flaccidity and tissue volume to be removed but is usually 4-6 cm. With the patient in the supine position, a bi-digital maneuver is performed and points D and E are marked in the inframammary fold (Figure 2).
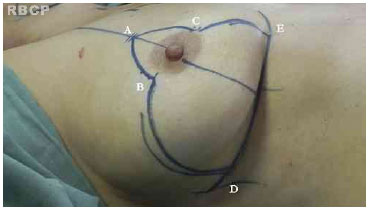
Figure 2. Pre-operative markings.
While marking the breast, the surgeon must consider the final breast volume desired by the patient, including the volume of the implant, to prevent excessive skin removal.
After the Schwartzman maneuver, breast tissue is removed from the lower pole and retroglandular detachment is performed (Figures 3A-C). Next, a type Pontes II complementary resection is performed at the posterior base of the breast (Figure 4)7. The breast pillar is approximated with nylon 3-0 and the implant is placed in the retroglandular position (Figures 5A, 5B). The NAC is repositioned and sutured in two layers using Monocryl® 4-0 in the subcutaneous and subdermal tissues and nylon 5-0 in the skin sutures (Figure 6). Drain placement is optional. The dressing is changed on the first postoperative day and the sutures are removed 7-10 days after surgery. A surgical bra is used for 30 days and Micropore surgical tape is placed for 60 days to prevent scar enlargement.
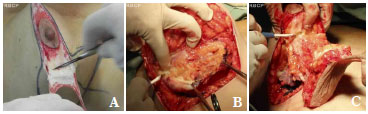
Figure 3. (A). Schwartzman maneuver. (B). Resection of the lower pole. (C). complementary resection of the base of the breast according to implant size.
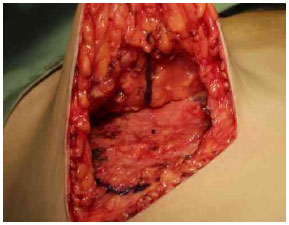
Figure 4. Resection of the base of the breast (Pontes II).
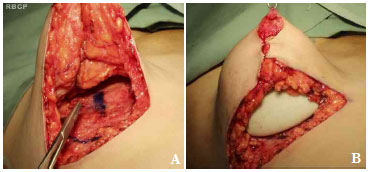
Figure 5. A. Approximation of the breast pillars. B. Placement of the retroglandular implant.
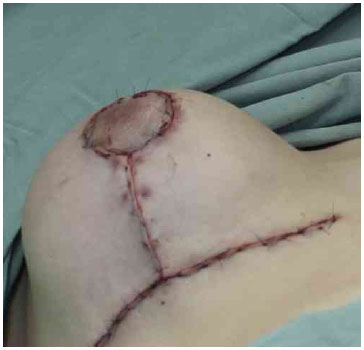
Figure 6. Final suture.
RESULTS
The average volume of breast tissue removed was 470 g on each side (range, 210-1,600 g). Carcinoma in situ was detected in two patients on histopathological examination. These two patients were regularly monitored and both were considered cured without the need for radiotherapy or chemotherapy. In most cases, improvement and maintenance of the projection of the upper pole as well as consistency and shape were observed with satisfactory results (Figures 7A-7C, pre-operative; Figures 8A-8C, post-operative; Figures 9A-C, pre-operative; and Figures 10A-10C, post-operative).
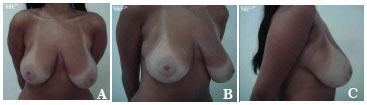
Figure 7. A-C. Pre-operative views.
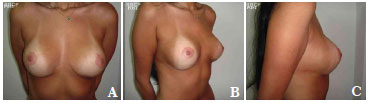
Figure 8. A-C. Post-surgical views after removal of 1,170 g of breast tissue plus the insertion of a round, high-profile, polyurethane implant (190 cc).
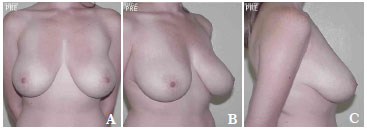
Figure 9. A-C. Pre-operative view.
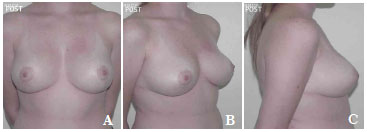
Figure 10. A-C. Post-surgical view after the removal of 590 g of breast tissue plus the insertion of a round, high-profile, polyurethane implant (190 cc).
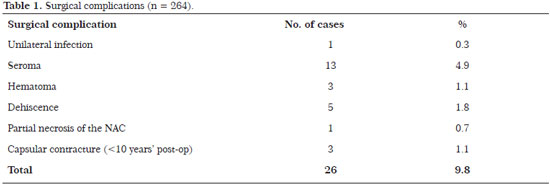
There were no significant complications such as flap or NAC necrosis. The incidence of complications was 9.8% (26 patients): one patient (0.3%) developed a unilateral infection that was treated with bilateral implant removal, drainage, and antibiotic therapy followed by the insertion of new implants 3 months later; three (4.9%) developed seroma that required surgical revision without the need for implant removalTimes New Roman three (1.1%) had hematoma that required drainage in the surgical centerTimes New Roman five (1.8%) had dehiscence, including one (0.7%) with partial NAC necrosis, requiring surgical review 6 months laterTimes New Roman and three (1.1%) developed capsular contracture before 10 years that required implant replacement (Table 1).
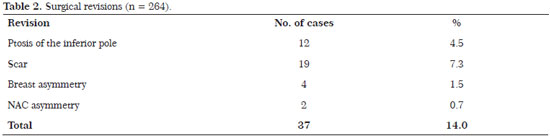
The revision rate was 17.8% (47 patients). The revisions were performed for the following reasons: 12 patients (4.5%) developed ptosis of the inferior pole; 11 (4.4%) were submitted to review of the scar; 4 (1.5%) with breast asymmetry; and 2 (0.7%) with asymmetry of the NAC. (Table 2)
DISCUSSION
The apparent paradox of the use of a silicone implant during a reduction mammoplasty can be better understood if we consider the tandem benefits of the two procedures. In Brazil, the decision to use implants in reduction mammoplasty is usually made by the patients. Their desires and expectations should be questioned and clarified to better inform them of the advantages and limitations of this association.
The long-term results with the implant seem to be better due to maintenance of the upper pole and breast consistency.
Patients with hypertrophy consisting of more adipose than glandular tissue, flaccid skin, loss of volume of the upper pole, and reduced consistency are the best candidates for the placement of small-volume implants during reduction mammoplasty. The indication for small-volume implants (190 cc) is due to the fact that augmentation mammoplasty is not indicated, so this leverages the existing breast tissue that can be added to the small prosthesis to create the final volume.
The long-term results are obtained due to the greater stability of the implant compared to the fatty tissue consistency. Technological advances currently offer breast implants that feature a lower risk of tissue reaction, which decreases the possibility of complications such as capsular contracture. The implants used can be textured or have polyurethane coverage, the latter being more adherent to tissue, producing good stability in the implant position and maintaining breast shape8.
The findings of carcinoma in situ in two patients merits attention. Tarone et al. reviewed five studies that observed the risk of breast cancer in patients who had undergone reduction mammoplasty. Follow-up studies in post-surgical women who underwent reduction mammoplasty indicate that the risk of cancer decreases in proportion to the increase in resected tissue9. The risk of breast cancer is reportedly lower in patients who underwent reduction mammoplasty compared to control patients10,11.
In addition to the oncological benefit, reduction mammoplasty results in functional improvements in musculoskeletal pain, headache, sleep, and breathing. Its psychological benefits include improved self-esteem, sexual function, and quality of life as well as reduced anxiety and depression. After reduction mammoplasty, women appear to exercise more and have a reduction in eating disorders12.
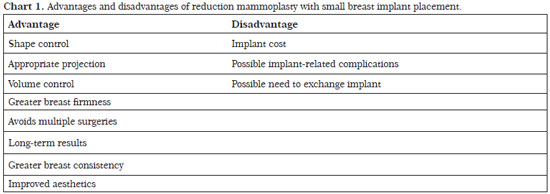
Reduction mammoplasty with breast implant placement is indicated for patients with moderate or severe flaccidity with any degree of hypertrophy as well as a greater fat than glandular content. Patients who previously underwent bariatric surgery and experienced significant weight loss can benefit from this technique. In our series, the rate of complications was acceptable and the long-term results were satisfactory for both patients and surgeons (Chart 1).
CONCLUSION
Consistent long-term results, improved aesthetics, and maintenance of the projection of the upper pole as well as breast shape and firmness can be obtained with the tandem use of reduction mammoplasty and placement of a small-volume breast implant. This procedure is especially indicated for patients with large amounts of flaccid skin, ptosis, and breast hypertrophy as well as in those whose breasts have higher fat than glandular content.
Reduction mastoplasty associated with silicone implant is a safe procedure in selected cases and features a high degree of patient and surgeon satisfaction.
REFERENCES
1. Schwartzman E. Die technik der mammaplastick. Chirurgie. 1930;2:932-43.
2. Lejour M. Vertical mammaplasty and liposuction of the breast. Plast Reconstr Surg. 1994;94(1):100-14.
3. Pitanguy I. Hipertrofias mamárias: estudo crítico da técnica pessoal. Rev Bras Cir. 1966;56:263.
4. Baroudi R, Lewis JR. The augmentation reduction mammaplasty. Clin Plast Surg. 1976;3(2):3018.
5. Saldanha OR. Uso de prótese em mamoplastia redutora. Arq Catarinenses Méd. 2000;29(supl.1):261.
6. Saldanha OR, Maloof RG, Dutra RT, Luz OA, Saldanha Filho O, Saldanha CB. Using prothesis in breast reduction. Rev Bras Cir Plast.2010;2:317-24.
7. Pontes R. Reduction Mammaplasty: Variations I and II. Annals Plast Surg.1981;6:437-447.
8. Pitanguy I, Amorim NG, Berfer AV. Analysis of implant exchange on the last five years at Ivo Pitanguy Clinic. Rev Bras Cir Plast.2010;4:668-674.
9. Tarone RE, Lipworth L, Young VL, McLaughlin JK. Breast Reduction Surgery and Breast Cancer Risk: Does Reduction Mammaplasty Have a Role in Primary Prevention Strategies for Women at High Risk of Breast Cancer? Plast Reconst Surg.2004;7:210-211.
10. Boice JD, Persson I, Brinton LA, Hober M, McLaughlin JK, Blot WJ, et al. Breast Cancer following Breast Reduction Surgery in Sweden. Plast Reconst Surg. 2000;4:755-762.
11. Brown MH, Weinberg M, Chong N, Levine R, Holowaty E. A Cohort Study of Breast Cancer Risk in Breast Reduction Patients. Plast Reconst Surg.1999;6:1674-1681.
12. Singh KA, Losken A. Additional Benefits of Reduction Mammaplasty: A Systematic Review of the Literature. Plast Reconst Surg. 2012;3:562-570.
1. MD, PhD, Division of Plastic Surgery, USP
2. MD, PhD, Division of Plastic Surgery, USP
3. MD - Resident of Plastic Surgery, Unisanta
4. MD - Resident of Plastic Surgery, Unisanta
5. MD - Resident of Plastic Surgery, Specialist in Plastic Surgery
6. MD - Resident in Plastic Surgery, Specialist in Plastic Surgery, Unimes
7. MD - Resident of Plastic Surgery, General Surgery Service, Sta Casa Santos
Institution: Clínica Saldanha.
Corresponding author:
Osvaldo Ribeiro Saldanha
Avenida Washington Luiz, 142
Santos, SP, Brazil Zip Code: 11.050-200
E-mail: clinica@clinicasaldanha.com.br
Article submitted: December 7, 2013.
Article accepted: November 25, 2014.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter