

Original Article - Year 2014 - Volume 29 -
Evaluation of the infection rate of implants used for breast reconstruction at the Cancer Institute of the State of São Paulo
Avaliação do índice de infecção de implantes mamários utilizados na reconstrução de mama do Instituto do Câncer do Estado de São Paulo
ABSTRACT
INTRODUCTION: Placement of breast implants is the most commonly used form of breast reconstruction. Despite its advantages, infection of the implant, either in the tissue expander or mammary prosthesis, can be a significant problem, including the need to remove it. The objective of this work is to evaluate the infection rate of breast implants used for breast reconstruction in patients submitted to surgery at the Cancer Institute of the State of São Paulo (ICESP), as well as its correlation with clinical, oncological, and surgical factors.
PATIENTS AND METHODS: This is a retrospective study on 120 patients submitted to breast reconstruction with breast implants at the ICESP from February 2009 to March 2010.
RESULTS: The infection rate (24.3%) was statistically related to immediate reconstruction (88.9%), diabetes mellitus (25%), body mass index >30 (52.8%), systemic arterial hypertension (52.8%), and skin injury due to mastectomy (27.8%). Of the infected implants, 44% were removed, most of which were expanders placed during immediate reconstruction.
CONCLUSIONS: Breast reconstruction with implants is the safest and most effective form of treatment. However, consideration should be given to patients who are prone to the development of infection, in order to optimize its prevention and attempt to perform its treatment at an early stage.
Keywords: Breast cancer; Expander/Breast implant; Infection.
RESUMO
INTRODUÇÃO: A utilização de implante mamário é a forma de reconstrução de mama mais comumente realizada. Apesar de suas vantagens, a infecção do implante, seja este expansor tecidual ou prótese mamária, pode ser um problema significativo, incluindo a necessidade de sua retirada. O objetivo deste trabalho é avaliar o índice de infecção de implantes mamários utilizados na reconstrução de mama de pacientes operadas no Instituto do Câncer do Estado de São Paulo (ICESP), bem como sua correlação com aspectos clínicos, oncológicos e cirúrgicos.
PACIENTES E MÉTODOS: Estudo retrospectivo de 120 pacientes submetidas à reconstrução mamária com implante mamário no ICESP, no período de fevereiro de 2009 a março de 2010.
RESULTADOS: O índice de infecção foi de 24,3% e esteve relacionado estatisticamente a reconstrução imediata (88,9%), diabetes mellitus (25%), IMC acima de 30 (52,8%), HAS (52,8%) e sofrimento de pele da mastectomia (27,8%). Nota-se que 44% dos implantes infectados foram retirados, sendo a maioria expansores colocados em reconstrução imediata.
CONCLUSÕES: A reconstrução mamária com implante é uma forma segura e eficaz de tratamento. Deve-se, entretanto, estar atento aos subgrupos de pacientes mais propensas ao desenvolvimento de infecção, para otimizar a sua prevenção e atentar ao seu tratamento precoce.
Palavras-chave: Câncer de mama; Expansor/Implante mamário; Infecção.
Placement of breast implants is the most commonly used form of breast reconstruction1,2. It has the advantage of being a simple procedure with low morbidity and short surgical time, in addition to promoting faster postoperative recovery and eliminating the morbidity of the donor area2,3. However, infection of the implant may be a significant problem that includes the need for implant removal, an increase in the number of surgical treatments, and a delay in oncological therapy4,5. Detecting and preventing this complication can reduce patient morbidity and hospital costs2.
The complications associated with breast reconstructions with alloplastic material and its relation to the clinical characteristics of the patient, and the surgical and oncological aspects of breast cancer treatment, are widely studied issues1,3,4,6-11. However, few studies have provided a clear and reproducible analysis. The definition of complication is also difficult to correlate between the published studies.
Only three works have addressed infection alone after placement of the breast implant. Olsen et al.9 comprehensively stratified the association between infection and several clinical characteristics of patients, tumor type, oncological therapy used, surgical aspects, and antibiotic prophylaxis used. However, this association also encompasses patients submitted to augmentation mastoplasty with aesthetic purposes. Nahabedian et al.12 did not differentiate between cases of immediate and late breast reconstructions, and did not perform statistical analysis of the infection rate or the clinical characteristics of the patients. Francis et al.2, in a retrospective study, did not include breast reconstructions with mammary implants, as well as some aspects related to the surgical procedure, in their analysis.
This study was conducted with the objective of assessing the infection rate of implants used for breast reconstruction in patients who underwent surgery at the Cancer Institute of the State of São Paulo (ICESP); diverse factors related to the clinical characteristics of patients, surgical technical aspects, methods of oncological treatment, and other postoperative complications were analyzed.
METHOD
Patients
This is a retrospective study on 120 female patients submitted to breast reconstruction with an expander and/or a breast implant at the ICESP, from February 2009 to March 2010, and who were followed for at least 1 year. The following data were collected: clinical characteristics and living habits of patients at the time of surgery, surgical-technical aspects related to resection and reconstruction, and the clinical oncological treatment implemented in each case and eventual postoperative complications followed by its treatment, with the aim of correlating these data to the infection rate in breast implants and identifying the most relevant aspects.
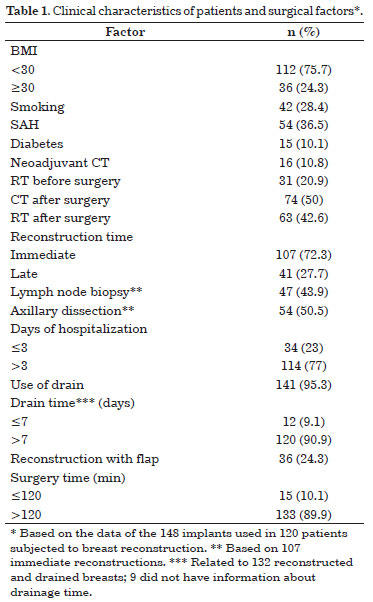
The clinical characteristics of the patients and the factors associated with the surgical procedure are described in Table 1. A total of 120 women were submitted to the placement of 148 expanders and/or mammary implants (99 expanders and 67 mammary implants). Patient ages ranged from 31 to 79 years, with an average of 51.9 years and a median of 52 years.
Surgical technique
Most patients were operated by the mastology team and received the clinical oncological treatment at the ICESP. However, some patients were directed from other institutions to follow clinical treatment (chemotherapy/radiotherapy) and surgical treatment (breast reconstruction).
The breast reconstructions were performed by the plastic surgery team of the hospital. The expanders used were the integrated-valve Mentor Siltex 6200 and Becker, selected according to the base of the breast of the patient. The implants used were also from the brand Mentor, anatomical (CPG), and the size was selected on the basis of the final expansion volume in comparison with, and on the basis of the height and base of the opposite breast.
The pocket of the expanders and/or implants was constructed in total retromuscular plane, through the dissection of the pectoralis major and serratus anterior muscles, or the myocutaneous rotation flap from the latissimus dorsi muscle. Before placement of the expander and/or implant, the pocket was cleaned with antibiotic solution (1 g cefazolin + 80 mg gentamicin + 100 mL physiological saline), and the skin was cleaned with alcoholic chlorhexidine solution. The expanders were filled with a solution containing 5 mL methylene blue and 35 mL physiological saline. The subsequent expanders were placed aseptically, in an outpatient setting, and the infused volume did not exceed 100 mL at each session. The time to exchange the expander for the implant was individualized. This procedure was performed at the end of the adjuvant therapy, taking into account the ideal expansion of the tissues. We adopted 6 months after the completion of radiotherapy as the minimum interval to exchange.
The drains were positioned at the subcutaneous and axillary pockets, during the concomitant axillary lymph node dissection, and removed as soon as the drainage volume was <30 mL/day.
Antibiotic prophylaxis was performed through the infusion of 2 g cefazolin during anesthetic induction, followed by an additional 1 g at every 3 h of surgery. Subsequently, therapy with first-generation cephalosporin was provided for a further 7 days.
Infection
Infection was defined according to the standardized criteria established by the Centers for Disease Control and Prevention (CDC)/National Healthcare Safety Network13, as the emergence of signs such as hyperemia, pain, swelling, induration, fever, secretion, fluid accumulation, dehiscence, and exposure of the expander and/or implant, during 1 year of postoperative monitoring.
Statistical analysis
Data were analyzed by using the program Epi Info version 7.0. The statistical p value was defined through the mid-p exact test and Fisher's exact test.
RESULTS
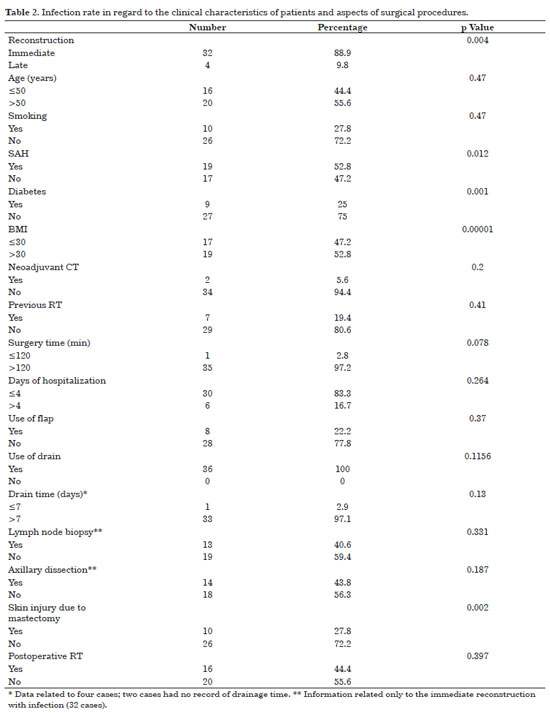
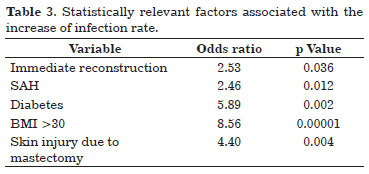
Infection occurred in 24.3% (n = 36) of the implants used, and its relation to the clinical characteristics of the patients and surgical aspects are presented in Table 2. Factors statistically related to infection are given in Table 3.
Among the 32 cases of infection that occurred in the immediate reconstruction, 28 occurred after the placement of the expander and 4 after the placement of the implant. Concerning late reconstruction (four cases of infection), infection occurred in the expander in one case and in the implant in three cases.
Age >50 years, smoking, arterial hypertension, diabetes, and body mass index (BMI) >30 presented associations with increased cases of infection; however, only the last three factors were statistically significant.
Two patients underwent neoadjuvant chemotherapy, and eight had already been subjected to radiation before surgery. Immediate reconstruction (85.7%) and skin injury due to mastectomy (25.7%) were related to the increase of infection rate, with a significant p value. Infection of the implant and/or expander occurred in most of the procedures that lasted for >2 h (97.2%), in 30 that required <3 days of hospitalization (83.3%), and in 8 surgeries where the flap was used to cover the implant and/or expander (22.2%). The drain was used in all procedures that progressed to infection and, from the 34 records that had information on drainage time, almost all (97.1%) were removed after 7 days. Lymph node biopsy was performed in 13 (37.1%) immediate reconstructions that progressed to infection, whereas axillary dissection was done in 12 (34.3%). Fourteen patients (40%) received postoperative radiotherapy. Table 3 shows the factors associated with infection that had statistical significance.
Infection was observed in three symmetrical breasts, with two occurring in the implanted breast and one concomitant with the infection of the contralateral reconstructed breast.
Seventeen implants and/or expanders (11.5% of the total placed) had to be removed, with 16 (10.8% of the total) because of infection and one owing to secondary extrusion and suture dehiscence. From the 16 cases that progressed to infection, two also showed extrusion of the expander and/or implant: one progressed in the same way due to necrosis of the latissimus dorsi flap, and in the other case, there was tumor recurrence with the need for new surgical intervention through mastology. It should be noted that, in another case, the infection occurred at 1 year and 4 months after the breast reconstruction and with the patient receiving adjuvant radiotherapy.
Adjuvant chemotherapy and radiotherapy were performed in all patients and in 14 patients who developed infection, respectively. The average time between surgery and the beginning of adjuvant oncological treatment in the patients who developed infection was of 3.5 months for chemotherapy and 8.9 months for radiotherapy.
Among the patients who had their implants removed because of infection, two (12.5%) received neoadjuvant chemotherapy, and one (6.3%) received radiotherapy before the surgery.
DISCUSSION
The indication for breast reconstruction is decided according to anatomical aspects, tumor extent, and preferences of both the patient and the surgeon. There are several postmastectomy reconstruction techniques, and the use of expanders and implants has been well accepted in the last 50 years. However, the risk of infection related to the expander and/or implant is well known and can occur at any time after the insertion.
The age of the patients (51.90 years) in this study reflects an increasingly younger population presenting with breast cancer, supporting various literature reports4,7. In addition, it has become increasingly common to perform the surgery to reduce the risk in patients at a high risk to develop breast cancer, which contributed to the increased number of breast reconstructions with implants. Although the infection rates of implants used in breast reconstructions were highly variable, the rate of 24.3% found in this work supports the data in the literature12,14,15, mainly owing to the high number of immediate reconstructions (72.3%). This type of reconstruction has become popular as it does not show damage in regard to oncological safety and surgical quality, besides contributing to the patient's self-esteem.
Most works found in the literature analyzed breast reconstructions with implants in relation to general complications but did not analyze infection separately. Besides infection, the following are also considered complications: seroma, hematoma, capsular contracture, loss of implant, necrosis of the skin after mastectomy, delay in surgical wound healing, and failure in expansion. As there are variations in regard to considering these events as complications, the comparison of our results with the analyses from the above-mentioned studies is hindered. Furthermore, it is important to underline that there are differences concerning the selection of patients and reconstruction type in the published works, which also affects the homogeneity of the results. Roostaeian et al.6 only assessed immediate reconstructions with breast prosthesis, and considered as complications skin necrosis after mastectomy, infection, hematoma, seroma, and capsular contracture, and did not show a relation between increase of complication risk and clinical aspects. McCarthy et al.4 analyzed all breast reconstructions with expander and/or implants performed from January 2003 to December 2004. These authors associated the increase in complication risk to age >65 years, hypertension, smoking, and BMI >30, and after including the following as complications: necrosis of the skin after mastectomy, seroma, hematoma, infection, failure in expansion, and exposure of the expander and/or implant. Crosby et al.7 assessed only immediate, unilateral breast reconstructions, and the use of the expander. They considered as complications the loss of the expander, infection, seroma, hematoma, skin necrosis after mastectomy, and delay in surgical wound healing. The authors found a relation between the increase of complication risk with factors such as prolonged time of drainage, axillary dissection, modified radical mastectomy (compared with simple mastectomy), and a high intraoperative expansion volume.
Few studies analyzed the infection of implants used in breast reconstruction as an isolated factor. Moreover, those studies primarily differ in the inclusion criteria of patients, which hinders the comparison between results. Francis et al.2 selected only immediate reconstructions with a tissue expander. Olsen et al.9 included all implants used in breast reconstruction and reduction mammaplasty. Nahabedian et al.12 selected their patients in a similar way to this study, considering patients receiving immediate and late breast reconstruction with an expander or an implant. However, they did not clarify the criteria used in their definition of infection. In our study, we considered as criteria of the diagnosis of infection those established in the consensus published in 2008 by the CDC13.
Infection was selected as an analysis factor in this work because it is the most frequent complication in breast reconstructions with alloplastic materials, and because it results in up to 40% removal of the material10.
The predictive factors of an increase in infection risk in breast reconstruction with alloplastic materials were immediate reconstruction, diabetes mellitus, BMI >30, systemic arterial hypertension, and skin injury after mastectomy. Approximately 44% (n = 16) of infected expanders/implants had to be removed, with most occurring in immediate reconstruction (n = 15) and after reconstruction with the breast expander (n = 14).
Nevertheless, in this work, a statistical relation was not found between infection and adjuvant oncological treatment. It is worth mentioning that patients were subjected to adjuvant treatment with chemotherapy, and that most patients (87.5%) who received radiotherapy after surgery developed infection of the expander/mammary prosthesis. Although controversial, several works showed that there is a significant increase in complication rates when these treatments are required6,11,12,16. In addition, it is important to underline that despite the infection, there was no delay in the beginning of adjuvant radiotherapy and chemotherapy. This is relevant because the ideal breast reconstruction method should not interfere with the oncological treatment.
Several factors have been reported to cause the increase of postoperative complications after the use of the expander and/or implant in breast reconstruction. However, few works have demonstrated the association of these factors with infection as an isolated result, and the difference in the method used in these studies prevents the reliable comparison of results. The detection of factors related to the increase of the infection rate may help in the selection of candidates for reconstructions with an expander and/or a breast implant, and thus, may prevent the premature removal of these materials as well as delays in the reconstruction.
We demonstrated that patients with a higher risk of infection after breast reconstruction with an alloplastic material are those subjected to immediate reconstruction, with diabetes mellitus, with BMI >30, hypertensive, and who develop skin injury after mastectomy. Special attention should be given to this subgroup of patients concerning the selected breast reconstruction technique.
REFERENCES
1. Serletti JM, Fosnot J, Nelson JA, Disa JJ, Bucky LP. Breast reconstruction after breast cancer. Plast Reconstr Surg. 2011;127(6):124e-35e. http://dx.doi.org/10.1097/PRS.0b013e318213a2e6. PMid:21617423
2. Francis SH, Ruberg RL, Stevenson KB, Beck CE, Ruppert AS, Harper JT, et al. Independent risk factors for infection in tissue expander breast reconstruction. Plast Reconstr Surg. 2009;124(6):1790-6. http://dx.doi.org/10.1097/PRS.0b013e3181bf80aa. PMid:19952635
3. Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg. 2006;118(4):825-31. http://dx.doi.org/10.1097/01.prs.0000232362.82402.e8. PMid:16980842
4. McCarthy CM, Mehrara BJ, Riedel E, Davidge K, Hinson A, Disa JJ, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121(6):1886-92. http://dx.doi.org/10.1097/PRS.0b013e31817151c4. PMid:18520873
5. Chun JK, Schulman MR. The infected breast prosthesis after mastectomy reconstruction: successful salvage of nine implants in eight consecutive patients. Plast Reconstr Surg. 2007;120(3):581-9. http://dx.doi.org/10.1097/01.prs.0000270296.61765.28. PMid:17700107
6. Roostaeian J, Pavone L, Da Lio A, Lipa J, Festekjian J, Crisera C. Immediate placement of implants in breast reconstruction: patient selection and outcomes. Plast Reconstr Surg. 2011;127(4):1407-16. http://dx.doi.org/10.1097/PRS.0b013e318208d0ea. PMid:21460648
7. Crosby MA, Dong W, Feng L, Kronowitz SJ. Effect of intraoperative saline fill volume on perioperative outcomes in tissue expander breast reconstruction. Plast Reconstr Surg. 2011;127(3):1065-72. http://dx.doi.org/10.1097/PRS.0b013e31820436fa. PMid:21364408
8. Pinsolle V, Grinfeder C, Mathoulin-Pelissier S, Faucher A. Complications analysis of 266 immediate breast reconstructions. J Plast Reconstr Aesthet Surg. 2006;59(10):1017-24. http://dx.doi.org/10.1016/j.bjps.2006.03.057. PMid:16996422
9. Olsen MA, Lefta M, Dietz JR, Brandt KE, Aft R, Matthews R, et al. Risk factors for surgical site infection after major breast operation. J Am Coll Surg. 2008;207(3):326-35. http://dx.doi.org/10.1016/j.jamcollsurg.2008.04.021. PMid:18722936
10. Yii NW, Khoo CT. Salvage of infected expander prostheses in breast reconstruction. Plast Reconstr Surg. 2003;111(3):1087-92. http://dx.doi.org/10.1097/01.PRS.0000046490.02212.BA. PMid:12621178
11. Mitchem J, Herrmann D, Margenthaler JA, Aft RL. Impact of neoadjuvant chemotherapy on rate of tissue expander/implant loss and progression to successful breast reconstruction following mastectomy. Am J Surg. 2008;196(4):519-22. http://dx.doi.org/10.1016/j.amjsurg.2008.06.016. PMid:18809054
12. Nahabedian MY, Tsangaris T, Momen B, Manson PN. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112(2):467-76. http://dx.doi.org/10.1097/01.PRS.0000070727.02992.54. PMid:12900604
13. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-32. http://dx.doi.org/10.1016/j.ajic.2008.03.002. PMid:18538699
14. Pittet B, Montandon D, Pittet D. Infection in breast implants. Lancet Infect Dis. 2005;5(2):94-106. http://dx.doi.org/10.1016/S1473-3099(05)01281-8. PMid:15680779
15. Hu YY, Weeks CM, In H, Dodgion CM, Golshan M, Chun YS, et al. Impact of neoadjuvant chemotherapy on breast reconstruction. Cancer. 2011;117(13):2833-41. http://dx.doi.org/10.1002/cncr.25872. PMid:21264833
16. Krueger EA, Wilkins EG, Strawderman M, Cederna P, Goldfarb S, Vicini FA, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49(3):713-21. http://dx.doi.org/10.1016/S0360-3016(00)01402-4. PMid:11172953
1. Specialist Member of the Brazilian Society of Plastic Surgery (SBCP), Assistant Physician at the Plastic Surgery Residency, University Hospital of São José (HUSJ), Faculty of Medical Sciences of Minas Gerais, Belo Horizonte, MG, Brazil
2.Associate Member of the Brazilian Society of Plastic Surgery (SBCP), Assistant Physician of the Subject Plastic Surgery at the Clínicas Hospital of the Faculty of Medicine, University of São Paulo (HCFMUSP/ICESP), São Paulo, SP, Brazil
3. Full Member of the Brazilian Society of Plastic Surgery (SBCP), Assistant Physician of the Subject Plastic Surgery at the Clínicas Hospital of the Faculty of Medicine, University of São Paulo (HCFMUSP/ICESP), São Paulo, SP, Brazil
4. Full Member of the Brazilian Society of Plastic Surgery (SBCP), Full Professor of the Subject Plastic Surgery, Clínicas Hospital at the Faculty of Medicine, University of São Paulo (HCFMUSP), São Paulo, SP, Brazil
5. Full Member of the Brazilian Society of Plastic Surgery (SBCP), PhD of the Surgical Clinics Program at the Faculty of Medicine, University of São Paulo (FMUSP), Coordinator of the Plastic Surgery Group at the Cancer Institute of the State of São Paulo (ICESP), University of São Paulo (USP), São Paulo, SP, Brazil
Institution: Work performed at the Cancer Institute of the State of São Paulo (ICESP), São Paulo, SP, Brazil.
Corresponding author:
Patrícia Noronha de Almeida
Faculdade de Ciências Médicas de Minas Gerais (FCMMG)
Rua Patagônia 285/202 - Sion
Belo Horizonte, MG, Brazil CEP 30320-080
E-mail: patynoronha@gmail.com
Article received: August 4, 2012.
Article accepted: October 15, 2012.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter