

Original Article - Year 2014 - Volume 29 -
Assessment of donor area pain in patients with cleft lip and palate undergoing alveolar bone defects repairs using iliac crest autogenous grafting: a prospective randomized comparison of two bone extractors
Avaliação da dor na área doadora de pacientes com fissura labiopalatina submetidos a reparo do defeito ósseo alveolar com enxerto autógeno de crista ilíaca: um estudo prospectivo randomizado comparando dois extratores ósseos
ABSTRACT
INTRODUCTION: Autogenous bone grafting is the standard treatment for alveolar bone defects. However, morbidity in the donor area after the bone graft has been obtained continues to be a significant problem in cleft patients. This prospective randomized study compared donor area pain associated with the use of 2 bone extractors in patients with cleft lip and palate, who underwent treatment of alveolar bone defects using a bone graft obtained from the iliac crest.
METHOD: Thirty-six patients with cleft lip and palate underwent alveolar bone defect repair using a graft from the iliac crest, harvested with either a SOBRAPAR bone extractor (group A) or UCLA bone extractor (group B). Donor area pain was evaluated in the postoperative period with the aid of a unidimensional numerical pain scale (0, "no pain"; 10, "worst pain imaginable").
RESULTS: Comparison of the mean donor area pain score did not reveal any significant differences (p >0.05 for all comparisons) between the groups A and B, at any of the postoperative times evaluated. A significantly higher number of patients in group B reported no pain in the donor area, compared with group A (p <0.05).
CONCLUSIONS: This study showed that a significantly greater number of patients in group B reported "no pain", compared with patients in group A; with regard to patients who reported any level of pain greater than zero, there were no between-group differences.
Keywords: Donor area; Iliac crest; Pain; Alveolar bone graft; Bone extractors; Cleft lip and palate.
RESUMO
INTRODUÇÃO: Enxerto ósseo autógeno é o padrão no tratamento da falha óssea alveolar. Como a morbidade na área doadora após a obtenção de enxerto ósseo continua sendo um problema relevante em pacientes fissurados, este estudo avaliou a dor na área doadora de pacientes fissurados submetidos ao tratamento de falhas ósseas alveolares com a transferência de enxerto ósseo obtido da crista ilíaca, por meio de um estudo prospectivo randomizado, comparando dois extratores ósseos.
MÉTODO: Trinta e seis pacientes com fissura labiopalatina, submetidos ao reparo da falha óssea alveolar com enxerto obtido da crista ilíaca com auxílio do extrator ósseo SOBRAPAR (grupo A) ou extrator ósseo UCLA (grupo B), foram incluídos. A dor na área doadora foi avaliada no período pós-operatório com auxílio da escala numérica unidimensional de dor (0- "sem dor"; 10- "pior dor que se pode imaginar").
RESULTADOS: As médias das mensurações da dor na área doadora não revelaram diferenças significativas (p>0,05 para todas as comparações) nas comparações realizadas entre os grupos A e B, em nenhum dos momentos pós-operatórios avaliados. Houve um maior número (p<0,05) de pacientes do grupo B que não reportaram dor na área doadora, quando comparado ao grupo A.
CONCLUSÕES: Este estudo apresentou um maior número de pacientes do grupo B "sem dor", quando comparado aos pacientes do grupo A, não existindo diferenças entre aqueles que reportaram quaisquer notas diferentes de zero.
Palavras-chave: Área doadora; Crista ilíaca; Dor; Enxerto ósseo alveolar; Extratores ósseos; Fissura labiopalatina.
Cleft lip and palate is the most common congenital craniofacial malformation, with an overall prevalence of 7.94 per 10,000 live births1. Among the numerous surgical interventions to treat these patients2,3, the repair of alveolar bone defects using an autogenous bone graft has been considered essential for rehabilitation, since it allows the restoration of the continuity of the maxillary arcade, the treatment of oronasal fistulas, appropriate dental eruption, and bone support for the alar base of the nose4.
Currently, grafting of autogenous bone removed from the iliac crest is considered the method of choice for the reconstruction of alveolar bone defects5. Historically, the iliac bone graft was obtained through an open approach (incision of 4 cm for viewing adequate tissue, dissection to the iliac crest, and obtaining bone with the aid of an osteotome), as described by Lindeman in 1915, and popularized by Wolfe & Kawamoto5,6. Currently, the harvest of bone grafts through a closed approach (minimum or minimally invasive access), using distinct surgical instruments (for example, percutaneous needle, Volkmann bone curette, modified trocar for bone biopsy, cylindrical osteotomes, among others), is performed in many centers of craniofacial plastic surgery4,5,7,8, including the bone extractor manufactured in our institution9.
In this context, it is important to mention that the harvest of bone grafts from the iliac crest is associated with numerous morbidities4,particularly pain in the donor area, which can be a source of anxiety, fear, and stress, for both the patients and their families10. Many international studies5,7,8,11-15 have assessed the differences between the open (traditional) and closed (minimally invasive access) approaches, and demonstrated lower morbidity rates, for examples, reduced donor area pain and gait disorders, in patients undergoing minimally invasive procedures. Following this trend, in 2004, our group9 demonstrated that the harvest of bone grafts from the iliac crest through the closed approach, with the aid of a bone extractor fashioned in our institution, was associated with a shorter duration of pain in the postoperative period, compared with the conventional open approach.
Despite these results5,7-9,11-15, to-date there has been no consensus about the ideal harvest technique by which to obtain autogenous bone to repair alveolar bone defects5,8. Thus, given that grafting autogenous bone is considered the standard method for the treatment of congenital bone defects, efforts should be focused on reducing the morbidity associated with this harvest 5,8. The objective of this study was to evaluate donor area pain reported by cleft lip and palate patients that underwent alveolar bone defect repair using bone grafts obtained from the anterior superior iliac crest. We utilized a prospective, randomized study design to compare 2 bone extractors used in the closed approach (minimally invasive access).
METHOD
A prospective randomized study was performed, involving 36 patients with cleft lip and palate that underwent alveolar bone grafting at the Institute of Craniofacial Plastic Surgery, Hospital SOBRAPAR, between October 2011 and April 2013. This study was approved by the Ethics Committee of Research in Humans of the Hospital SOBRAPAR, and is in accordance with the Declaration of Helsinki in 1975, revised in 1983.
We included patients with complete unilateral non-syndromic cleft lip and palate, undergoing transfer of secondary alveolar bone grafting (aged between 7 and 12 years) or late secondary alveolar bone grafting (aged >12 years)4. Only patients with grafts harvested from the anterior superior iliac crest by a minimally invasive access (closed approach with bone extractors), who attended postoperative follow-up, and who agreed to participate in the study by providing informed written consent, were included. All patients with a medical or surgical history that could interfere with the assessment of pain in the postoperative period (for example, cognitive deficits, chronic use of analgesic and/or anti-inflammatory medications, presence of any surgical interventions in the anterior superior iliac crests region or chronic pain disorders16) were excluded. Patients are at risk of a state of acute confusion in the immediate postoperative period, which can affect the ability to grade their pain16; patients were therefore not evaluated in this period in our study.
Bone extractors
In the SOBRAPAR hospital, bone grafts from the iliac crest have been preferentially harvested with the aid of 2 metallic cylindrical manual bone extractors. One of these extractors was produced in our institution (Hospital SOBRAPAR, Campinas, Brazil) and has been named the SOBRAPAR bone extractor9, while the other was manufactured at the University of California, Los Angeles (UCLA, California, United States), and is named the UCLA bone extractor (Figure 1). Both surgical instruments are made from similar materials (surgical steel of the same thickness); the main difference between them is the diameter of the cylinder (SOBRAPAR bone extractor, 0.8 cm; UCLA bone extractor, 0.5 cm) (Figure 2).

Figure 1. Manual cylindrical metallic bone extractors produced at the SOBRAPAR Hospital (left) and at the University of California, Los Angeles (UCLA) (right). Both instruments have a cylindrical metal rod with a sharp edge; the other end is connected to a "T" cable (UCLA extractor) or a "circular" cable (SOBRAPAR extractor), which allows a firm grip without release during the bone harvest from the anterior superior iliac crest. Note the metal pistons, used to remove bone from the interior of the extractors.
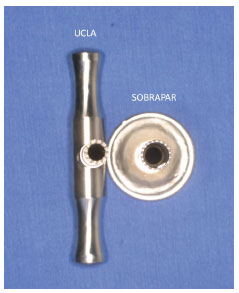
Figure 2. Manual cylindrical metallic bone extractors produced at the University of California, Los Angeles (UCLA) (left) and SOBRAPAR Hospital (right). Note the difference between the diameters (SOBRAPAR extractor, 0.8 cm) and (UCLA extractor, 0.5 cm).
Harvesting bone grafts from the iliac crest using a minimally invasive approach
Before surgery, patients were randomly distributed into 2 groups: patients submitted to harvest of iliac crest bone grafts with the SOBRAPAR bone extractor (group A), or with the UCLA bone extractor (group B).
All surgical interventions for the harvest of bone grafts from the iliac crest were standardized, and performed by one of 3 plastic surgeons, with the same training and philosophy, with the aid of a resident. This procedure is considered minimally invasive when compared to the traditional technique, because it uses very similar steps to those employed in the open approach, with the exception of a smaller incision, and minimal tissue dissection/manipulation (closed approach or minimally invasive access)7.8.
The surgeries were performed under general anesthesia. In group A, a 2 cm incision was made obliquely, below the anterior superior iliac crest. Minimum tissue was dissected until the periosteum of the iliac crest was reached. Thereafter, a minimal amount of subperiostal detachment was performed, up to at least 3 cm deep at the more superficial point of the anterior superior iliac crest, such that the presence of bone could be detected by applying slight pressure to the instrument against the anatomical structure. The rotatory movements of the extractor were performed until to the absence of resistance (Figure 3). In group B, the same incision allowed the loading of the bone extractor on the surface of the iliac crest without periosteal detachment and withdrawal of cartilage and bone marrow (Figure 4). The block of cartilage was subsequently returned, and only the medullary bone was used. A metal plunger was applied to remove the bone from the inside of the extractor. This was followed by hemostasis and closure in layers.
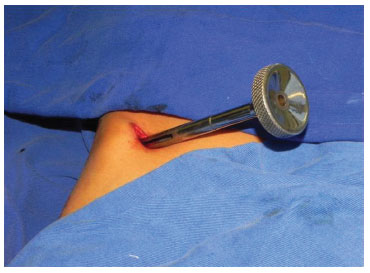
Figure 3. Manual cylindrical metallic bone extractor produced in the SOBRAPAR Hospital; shown in situ during bone harvest from the anterior superior iliac crest.
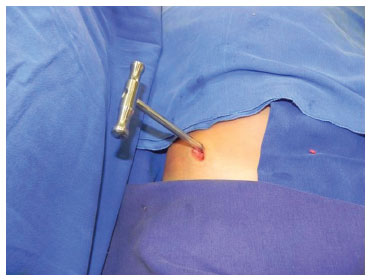
Figure 4. Manual cylindrical metallic bone extractor produced at the University of California, Los Angeles (UCLA); shown in situ during bone harvest from the anterior superior iliac crest.
Care in the postoperative period and analgesia
Care in the postoperative period was similar for all patients, and consisted of early mobilization, with assistance if necessary, and guidance on daily care of the surgical wound. Stitches were removed on postoperative day 7. All patients were discharged from hospital on the first postoperative day. Patients were advised to abstain from their usual activities, for example, school or work, for 2 weeks, and to avoid any physical activity for 6 weeks10,17,18.
Postoperative analgesia comprised only non-narcotic analgesics (dipyrone sodium 500 mg/cp, or paracetamol 750 mg/cp if allergic to dipyrone); analgesia was continued for the duration of the individual's complaints, in accordance with their needs5,19. No analgesic or anti-inflammatory medication was routinely used. There was no use of local anesthetics, epidural, or locoregional analgesia, in any of the procedures included in this analysis16.
Assessment of pain
Similarly to other studies5,19 on alveolar bone grafting, the intensity of donor area pain was measured in all patients included in the study, according to a unidimensional numerical pain scale20, which has been proven to be valid and reliable in both young and adults16,20. This scale is composed of 11 numbers (0-10), distributed at equal distances in a straight line, with '0' characterized as "no pain" and '10' as "worst pain imaginable" 16,20.
Before the application of the scale, a responsible researcher read aloud the standardized instructions to the patient. Patients were then asked to rate the current level of donor area pain16,20. Patients marked the figures corresponding to their level of donor area pain at 10 different times during the postoperative period: 1, 3, 6, 9 and 12 hours after the procedure, and on the 3rd, 7th, 14th, 21st, and 28th postoperative days21. The evaluation of pain on the part of the patients was independent of the researcher in charge; this researcher did not participate in any surgical procedure, and had no knowledge of the study groups5,19.
Statistical analysis
All information was compiled in Excel 2013 for Windows (Microsoft Corporation, USA). For the descriptive analysis, the mean was used for metric variables, and percentages for categorical variables. The simple mathematical difference of each postoperative time point, when compared with the first assessment undertaken at 1 hour postoperatively, was calculated to characterize change in the results (reduction, increase, or maintenance). Two tests (ANOVA and equality of 2 proportions) and the confidence interval for the mean were used for all comparative analyses between groups A (SOBRAPAR bone extractor) and B (UCLA bone extractor). The programs Statistical Package for Social Sciences version 17 (SPSS, Chicago, IL, USA) and Minitab version 16 (Minitab, Inc., USA) were used for the statistical analyses. The values were considered significant for a confidence interval of 95% (p <0.05).
RESULTS
A total of 32 (88.89%) patients met the inclusion criteria (p <0.05). Four patients were excluded (11.11%; group A, 3 patients; group B, 1 patient), because they did attend follow-up on the days specifically scheduled for the assessment of donor area pain. Group A (SOBRAPAR bone extractor) comprised 15 patients (46.88%), while group B (UCLA bone extractor) comprised 17 patient (53.12%) (p = 0.617). The mean age of patients at the time of the surgical intervention was 15.06 ± 7.30 years (group A, 15.31 ± 6.78 years; group B, 14.81 ± 8.00 years; p >0.05). Twenty-one (65.63%) patients were male (group A, 10 patients; group B, 11 patients; p >0.05) and 11 (34.37%) were female (group A, 6 patients; group B, 5 patients; p >0.05).
Assessment of pain
The intensity of donor area pain was reported as 0 ("no pain") during all evaluation periods by 6 (18.75%) patients, while a significant majority (p <0.001; 26 patients, 81.25%) reported donor area pain intensity of between 0 ("no pain") and 7, depending on the time of evaluation (Figure 5). No patients graded a pain intensity of >8. Considering the total number of patients (n = 32), a comparative analysis between the groups revealed no significant difference in the proportion of patients that reported pain intensity in the donor area as 0 ("no pain") at all evaluation periods (group A, 1 patient, 6.67%; group B, 5 patients, 29.41%; p >0.100). Analysis of the subgroup of patients (n = 6) who did not report donor area pain (grade 0, "no pain"), there was a significant predominance (p = 0.021) of group B patients (5 patients, 83.33%), compared with group A (1 patient, 16.67%).
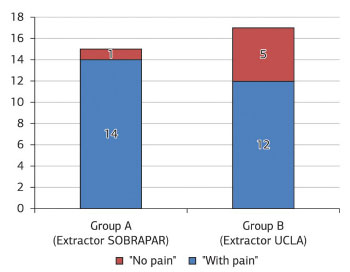
Figure 5. Distribution of patients with cleft lip and palate (n = 32) who underwent repair of bone defect with alveolar bone graft from the iliac crest obtained with the SOBRAPAR bone extractor (group A) or UCLA (University of California, Los Angeles) bone extractor (group B), according to the presence (n = 26) or absence (n = 6) of donor area pain.
The mean measures of donor area pain by means of the unidimensional numerical pain scale did not differ significantly between groups (p >0.05 for all comparisons) at any of the postoperative evaluation periods (Table 1 and Figure 6). The analysis comparing values obtained between the postoperative evaluation periods and the first postoperative evaluation (1 hour postoperatively) also revealed no significant differences (p >0.05 for all comparisons) (Table 2 and Figure 7). All patients reported the intensity of donor area pain as 0 ("no pain") after the 14th postoperative day.
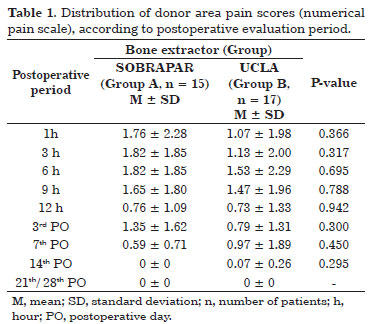
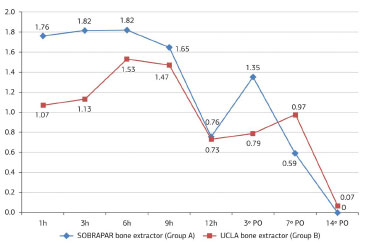
Figure 6. Distribution of the mean donor area pain scores (numerical pain scale), according to the postoperative period (PO). Note the trend toward a reduction in the mean values in both groups.
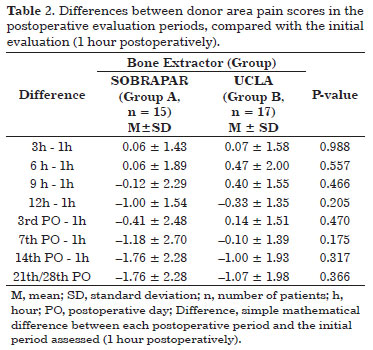
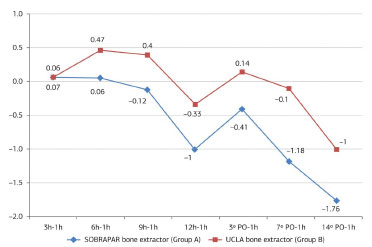
Figure 7. Distribution of the values obtained with the calculation of the simple mathematical difference (postoperative period (PO), in relation to the initial evaluation period (1 hour postoperatively)). Note the trend toward a reduction in the difference in both groups).
DISCUSSION
The repair of alveolar bone defects using autogenous bone was introduced at the beginning of the 20th century4. However, this procedure only became widely-used after the publication of Boyne and Sandys22 over alveolar bone grafts in cleft patients with mixed dentition. In the SOBRAPAR hospital, and in many other centers of craniofacial plastic surgery2,3,5, patients with a cleft lip and palate have been preferably treated with alveolar bone grafting immediately before the eruption of the canine teeth (aged 7-12 years of age), with prior monitoring and orthodontic treatment.
However, in accordance with another analysis23, patients undergoing late alveolar bone grafting (aged >12 years) were also included in the present study. As such, the average age of the patients included is larger than that reported previously5,17. This is likely a result of the intrinsic characteristic of our service, which acts as one of the national referral centers for the treatment of craniofacial deformities, including cleft lip and palate, and receives sequelae patients, often outside the age range considered ideal for the execution of a certain surgical procedure2.
The iliac crest has been the donor area of choice in the treatment of alveolar bone defects4,5,8,10, even if there are many other potential donor sites4. In general, one can obtain an iliac bone graft through open (traditional) or closed (minimally invasive access) surgical interventions5,6. Based on numerous studies5,7-9,11-15 that have shown a lower rate of morbidity associated with the use of closed interventions, our group and others5 have adopted minimally invasive access as the method of choice for the harvesting bone grafts from the iliac crest, for use in correct alveolar bone repairs. However, there are many tools available for the minimally invasive harvest of autogenous bone4,5,7,8 and there is no standardization on the choice between these tools5,8.
In this context, unlike other centers5 that use only one electrical instrument, our group has used 2 different bone extractors; one produced in our institution9 and another produced at UCLA. In Brazil, as the costs of the specialized centers for the treatment of craniofacial deformities are only partially financed (50-60%) by the Single Health System (SUS)2, any factors that might have an impact on the overall cost of the treatment of cleft lip and palate patients should be considered in the choice between different surgical materials. Thus, we have adopted both extractors due to low cost of the acquisition and maintenance of these tools, when compared, for example, with electrical industrial instruments that have greater financial costs5. As with other instruments24, both extractors used by us are simple to assemble, use, and sterilize, particularly in comparison with multiple component systems5,24. Another relevant factor is durability, because like other instruments24, the SOBRAPAR and UCLA extractors can be used in numerous procedures without the need for replacement; this is in contrast with the Acumed power-driven trephine system, for example, which needs to be replaced every 10-20 patients5.
The data from this study indicated that there were no statistically significant differences between the bone extractors used. We believed that this would be a consequence of the differences between the extractors; the larger diameter of SOBRAPAR bone extractor (0.8 cm), and requirement for additional manipulation of the periosteum, compared with the UCLA bone extractor (0.5 cm diameter, no requirement for subperiosteal detachment). However, we did not have any specific data to support this hypothesis or to support a choice between the bone extractors. Thus, the present study was designed to compile specific information relating to donor area pain in the postoperative period, after the use of either one of these 2 bone extractors, with the ultimate goal of obtaining evidence to support decisions related to the use of each instrument. Another group8 also conducted a study to compare 2 instruments used to harvest autogenous bone through minimally invasive access. However, pain reported by patients was not measured based on a previously validated scale8.
Assuming that pain was not measured on the basis of any established system, limitations must be established for the results on pain associated with the use of the SOBRAPAR bone extractor obtained in our preliminary study9. In order to remove this limitation in the present study, we ensured that we adopted a unidimensional scale of pain intensity (numerical pain scale), that has been used in similar analyzes5,19. In the literature, different scales have been applied for the measurement of pain, ranging from complex multidimensional instruments, to very simple numerical16,20 scales. It is important to note that the scale used as a grading tool in the present study (numerical pain scale) is the pain intensity scale preferred by patients and physicians, due to its relative simplicity and ease of administration/scoring16,20. Moreover, the numerical pain scale has a low error rate and higher validity when compared with other unidimensional pain intensity scales such as, for example, the visual analog scale 16.
In the present study, the majority of patients (81.25%) reported some degree of donor area pain. In similar studies21,25,26, the incidence of pain in the donor area (iliac crest) varies widely, between 2.8% and 100%; the various methods adopted for the measurement of donor area pain are among the main reasons for this great discrepancy26. Thus, there are limitations as to comparisons that can be made between the results obtained in different studies. We will, therefore, only offer a few comments on the numerical data, because any in-depth comparative analysis would be merely speculative21.
It is important to establish that there are differences between the measurement of acute pain, which persists for days/weeks and regresses during the recovery process, and chronic pain, which continues after tissue injury has been repaired16.21. As in another study21, the results of the present study demonstrated changes in the perception of donor area pain over time. In other words, the patients measured their pain, on average, as less than 2, and this intensity displayed a tendency to decrease, as illustrated in Figure 6. In addition, all patients in our study who reported some degree of pain (81.25% of the total number of patients), showed complete resolution of the symptom ("no pain", grade 0) after the 14th postoperative day; therefore, there were no cases of chronic pain, as in a similar analysis10. Conversely, in previous studies21,25,27 the period of complete resolution of donor area pain was variable (weeks to months) and, in some situations, the incidence of chronic pain reached 33% at 12 months postoperatively21.
The results of the present study demonstrated that the intensity of donor area pain in the postoperative period, in patients submitted to minimally invasive surgeries to harvest bone grafts from the iliac crest, did not differ depending on whether the SOBRAPAR bone extractor (group A), or the UCLA bone extractor (group B) was used. Although the trends shown in Figures 6 and 7 indicate differences between groups A and B, particularly during the first 12 hours postoperatively, the statistical analysis showed that there were no differences between the 2 extractors. It is plausible that the small sample size may have biased these results, as emphasized in a similar analysis8.
In the previous literature, approximately two-thirds of patients reported no donor area pain26. In our study, 18.75% of patients graded the pain in the donor area as 0 ("no pain") at all evaluation time periods. Interestingly, among those patients with "no pain" there was a significant predominance of group B patients (UCLA bone extractor). These data support our initial hypothesis; however, the reason that some patients graded pain in the donor area as 0 ("no pain") and the rest of the patients in the same group presented variable intensities of pain (grades 1 and 7) is not clear, because the surgical techniques and postoperative care were standardized both in our study and in another26 which reported similar results. Indeed, the exact etiology of pain in the donor area (iliac crest) remains obscure25. It has been postulated that pain has its origin in the manipulation of the muscle and/or the periosteum; alternatively it may be secondary to sensory nerve injury25. We and others26 believe that the cause of pain in the donor area (iliac crest) should be considered multifactorial, and is probably related to a combination of muscle/periosteal manipulation, injury to sensory nerves, and emotional factors. Therefore, in addition to the diameter of the bone extractor and tissue manipulation, other aspects may have influenced the results of the present study.
Although the surgical manipulation techniques of both bone extractors are distinct, it was possible to conclude, from the data of the present study, that the grading of pain in patients undergoing harvesting of bone from the iliac crest, was similar. As the amount of bone removed in each surgical approach with both bone extractors is similar, we use the bone extractors in our practice at random. Interestingly, the position of the UCLA extractor is always perpendicular to the abdominal region, while the SOBRAPAR extractor is always oblique to the abdominal region. As bone graft harvest requires the application of pressure to the end of the extractor, the use of the UCLA extractor may lead more easily to perforating accidents, mainly in the hands of less experienced surgeons. Therefore, before using the UCLA, the first-year resident spends approximately one year using the SOBRAPAR bone extractor. With this care, we promote effectiveness and safety in the surgical procedure.
The present study has some limitations that should be addressed. Although patient-reported pain was the ultimate concern of our study, other forms of analysis related directly or indirectly to donor area pain, for example, quantification of the use of analgesic medications, have been applied in similar studies5,19. The limitations of the use of different methods should be considered in the interpretation of the results. In addition, it has been demonstrated that the removal of large amounts of bone graft from the iliac crest is not associated with increased pain in the donor area postoperatively26. However, we did not measure the amount of bone from the iliac crest and, therefore, specific limitations as to the amount of bone obtained related to the 2 instruments evaluated should be imposed. The complications/comorbidities associated with the harvesting of autogenous bone grafts are well-established, largely by the group led by Dr. Paul Tessier28, which published a seminal article based on the experience of 20,000 procedures performed over 50 years. Our main interest in the present study was to concentrate exclusively on the evaluation of donor area pain; we therefore took no additional records, nor performed any analysis, on any other associated morbidity. Thus, another limitation is that this study only evaluated one aspect related to the morbidity of the donor area, and this should also be considered when any conclusions are drawn from the results presented.
This research was prospective in nature, the patients were appropriately randomized and used a previously validated and widely-used pain scale16,20; we therefore believe that the above limitations do not invalidate the results. However, future research should be conducted to examine our findings, and also to address the limitations of our study, with the aim of enriching our understanding of harvesting bone grafts from the iliac crest through a minimally invasive access using bone extractors.
CONCLUSION
This prospective randomized study evaluated donor area (iliac crest) pain in the postoperative period. The results demonstrated that a greater number of patients undergoing bone harvest with the UCLA bone extractor (group B) graded the pain as 0 ("no pain"), compared with group A patients (SOBRAPAR bone extractor); however, there were no significant differences between those patients that reported any grading different from zero.
REFERENCES
1. Tanaka SA, Mahabir RC, Jupiter DC, Menezes JM. Updating the epidemiology of cleft lip with or without cleft palate. Plast Reconstr Surg. 2012;129(3):511e-8e. http://dx.doi.org/10.1097/PRS.0b013e3182402dd1.PMid:22374000
2. Raposo-Amaral CE, Raposo-Amaral CA. Changing face of cleft care: specialized centers in developing countries. J Craniofac Surg. 2012;23(1):206-9. http://dx.doi.org/10.1097/SCS.0b013e318241ae70. PMid:22337409
3. Alonso N, Tanikawa DYS, Lima Junior JE, Ferreira MC. Avaliação comparativa e evolutiva dos protocolos de atendimento dos pacientes fissurados. Rev Bras Cir Plast. 2010;25(3):434-8. http://dx.doi.org/10.1590/S1983-51752010000300006.
4. Bajaj AK, Wongworawat AA, Punjabi A. Management of alveolar clefts. J Craniofac Surg. 2003;14(6):840-6. http://dx.doi.org/10.1097/00001665-200311000-00005. PMid:14600625
5. Sharma S, Schneider LF, Barr J, Aarabi S, Chibbaro P, Grayson B, et al. Comparison of minimally invasive versus conventional open harvesting techniques for iliac bone graft in secondary alveolar cleft patients. Plast Reconstr Surg. 2011;128(2):485-91. http://dx.doi.org/10.1097/PRS.0b013e31821b6336. PMid:21788839
6. Wolfe SA, Kawamoto HK. Taking the iliac-bone graft. J Bone Joint Surg Am. 1978;60(3):411. PMid:348708.
7. Gimbel M, Ashley RK, Sisodia M, Gabbay JS, Wasson KL, Heller J, et al. Repair of alveolar cleft defects: reduced morbidity with bone marrow stem cells in a resorbable matrix. J Craniofac Surg. 2007;18(4):895-901. http://dx.doi.org/10.1097/scs.0b013e3180a771af. PMid:17667684
8. Burstein FD, Simms C, Cohen SR, Work F, Paschal M. Iliac crest bone graft harvesting techniques: a comparison. Plast Reconstr Surg. 2000;105(1):34-9. http://dx.doi.org/10.1097/00006534-200001000-00006. PMid:10626967
9. Silva WL Jr, Buzzo CL, Módolo JH, Basso RC, Reda AL, Lopes RG, et al. Estudo comparativo para extração óssea em enxerto alveolar. Rev Soc Bras Cir Craniomaxilofac. 2004;7(2):16.
10. Becker ST, Warnke PH, Behrens E, Wiltfang J. Morbidity after iliac crest bone graft harvesting over an anterior versus posterior approach. J Oral Maxillofac Surg. 2011;69(1):48-53. http://dx.doi.org/10.1016/j.joms.2010.05.061. PMid:20971544
11. McCanny CM, Roberts-Harry DP. A comparison of two different bone-harvesting techniques for secondary alveolar bone grafting in patients with cleft lip and palate. Cleft Palate Craniofac J. 1998;35(5):442-6. http://dx.doi.org/10.1597/1545-1569(1998)035<0442:ACOTDB>2.3.CO;2. PMid:9761565
12. Hardy SP, Wilke RC, Doyle JF. Advantages of percutaneous hollow needle technique for iliac bone harvest in alveolar cleft grafting. Cleft Palate Craniofac J. 1999;36(3):252-5. http://dx.doi.org/10.1597/1545-1569(1999)036<0252:AOPHNT>2.3.CO;2. PMid:10342614
13. Eufinger H, Leppänen H. Iliac crest donor site morbidity following open and closed methods of bone harvest for alveolar cleft osteoplasty. J Craniomaxillofac Surg. 2000;28(1):31-8. http://dx.doi.org/10.1054/jcms.2000.0105. PMid:10851671
14. Sàndor GK, Nish IA, Carmichael RP. Comparison of conventional surgery with motorized trephine in bone harvest from the anterior iliac crest. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(2):150-5. http://dx.doi.org/10.1067/moe.2003.42. PMid:12582353
15. Constantinides J, Chhabra P, Turner PJ, Richard B. A comparison of Shepard's osteotome versus trapdoor flap technique to harvest iliac crest bone for secondary alveolar bone grafting. Cleft Palate Craniofac J. 2008;45(4):347-52. http://dx.doi.org/10.1597/06-235.1. PMid:18616364
16. Gagliese L, Weizblit N, Ellis W, Chan VW. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117(3):412-20. http://dx.doi.org/10.1016/j.pain.2005.07.004. PMid:16153776
17. Swan MC, Goodacre TE. Morbidity at the iliac crest donor site following bone grafting of the cleft alveolus. Br J Oral Maxillofac Surg. 2006;44(2):129-33. http://dx.doi.org/10.1016/j.bjoms.2005.04.015. PMid:15961201
18. Matsa S, Murugan S, Kannadasan K. Evaluation of morbidity associated with iliac crest harvest for alveolar cleft bone grafting. J Maxillofac Oral Surg. 2012;11(1):91-5. http://dx.doi.org/10.1007/s12663-011-0249-2. PMid:23449884
19. Dashow JE, Lewis CW, Hopper RA, Gruss JS, Egbert MA. Bupivacaine administration and postoperative pain following anterior iliac crest bone graft for alveolar cleft repair. Cleft Palate Craniofac J. 2009;46(2):173-8. http://dx.doi.org/10.1597/07-136.1.PMid:19254053
20. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399-404. http://dx.doi.org/10.1016/j.pain.2011.07.005. PMid:21856077
21. Barone A, Ricci M, Mangano F, Covani U. Morbidity associated with iliac crest harvesting in the treatment of maxillary and mandibular atrophies: a 10-year analysis. J Oral Maxillofac Surg. 2011;69(9):2298-304. http://dx.doi.org/10.1016/j.joms.2011.01.014. PMid:21470738
22. Boyne PJ, Sands NR. Secondary bone grafting of residual alveolar and palatal clefts. J Oral Surg. 1972;30(2):87-92. PMid:4550446.
23. Baqain ZH, Anabtawi M, Karaky AA, Malkawi Z. Morbidity from anterior iliac crest bone harvesting for secondary alveolar bone grafting: an outcome assessment study. J Oral Maxillofac Surg. 2009;67(3):570-5. http://dx.doi.org/10.1016/j.joms.2008.09.023. PMid:19231782
24. Cobb AR, McCarthy E, Van Zyl M, Ayliffe PR. Alveolar bone grafting: use of the Jacob's chuck with trephine to harvest iliac crest cancellous bone. Br J Oral Maxillofac Surg. 2011;49(3):239-40. http://dx.doi.org/10.1016/j.bjoms.2010.04.013. PMid:20546982
25. Joshi A, Kostakis GC. An investigation of post-operative morbidity following iliac crest graft harvesting. Br Dent J. 2004;196(3):167-71, discussion 155. http://dx.doi.org/10.1038/sj.bdj.4810945. PMid:14963443
26. Heary RF, Schlenk RP, Sacchieri TA, Barone D, Brotea C. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery. 2002;50(3):510-6, discussion 516-7. PMid:11841718.
27. Rawashdeh MA. Morbidity of iliac crest donor site following open bone harvesting in cleft lip and palate patients. Int J Oral Maxillofac Surg. 2008;37(3):223-7. http://dx.doi.org/10.1016/j.ijom.2007.11.009. PMid:18272337
28. Tessier P, Kawamoto H, Posnick J, Raulo Y, Tulasne JF, Wolfe SA. Complications of harvesting autogenous bone grafts: a group experience of 20,000 cases. Plast Reconstr Surg. 2005;116(5 Suppl):72S-3S, discussion 92S-4S. http://dx.doi.org/10.1097/01.prs.0000173841.59063.7e.PMid:16217446
1. MD, ex-resident, "Prof. Dr. Cassio M. Raposo do Amaral" Plastic Surgery Service, Craniofacial Plastic Surgery Institute, Hospital SOBRAPAR, Campinas, SP, Brazil
2. MD, Aspiring member in Training of the Brazilian Society of Plastic Surgery (SBCP), Resident Physician in Plastic Surgery, "Prof. Dr. Cassio M. Raposo do Amaral" Plastic Surgery Service, Craniofacial Plastic Surgery Institute, Hospital SOBRAPAR, Campinas, SP, Brazil
3. MD, MSc, Full Member of the Brazilian Society of Plastic Surgery (SBCP)and the Brazilian Association of Cranio-Maxillo-Facial Surgery (ABCCMF), MSc in Plastic Surgery by the Universidade Estadual de Campinas (UNICAMP), Regent of the "Prof. Dr. Cassio M. Raposo do Amaral" Plastic Surgery Service, Craniofacial Plastic Surgery Institute, Hospital SOBRAPAR, Campinas, SP, Brazil
4. MD, PhD, Full Member of the Brazilian Society of Plastic Surgery (SBCP) and the Brazilian Association of Cranio-Maxillo-Facial Surgery (ABCCMF), PhD by the Clinical Surgery Program, Universidade de São Paulo (USP), Vice-president of the Craniofacial Plastic Surgery Institute, Hospital SOBRAPAR, Campinas, SP, Brazil
5. MD, Full Member of the Brazilian Society of Plastic Surgery (SBCP), Head Tutor of the Residents, "Prof. Dr. Cassio M. Raposo do Amaral" Plastic Surgery Service, Craniofacial Plastic Surgery Institute, Hospital SOBRAPAR, Campinas, SP, Brazil
Institution: Study conducted at the Institute of Craniofacial Plastic Surgery, Hospital SOBRAPAR, Campinas, SP, Brazil.
Corresponding author:
Cesar Augusto Raposo-do-Amaral
Hospital de Crânio e Face SOBRAPAR
Av. Adolpho Lutz, 100 - Cidade Universitária
Campinas, SP, Brazil CEP 13083-880; Caixa-postal 6028
E-mail: cesaraugustoraposo@hotmail.com
Article received: January 22, 2014.
Article accepted: August 03, 2014.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter