

Original Article - Year 2014 - Volume 29 -
Technical standardization of the microsurgery training program of plastic surgery and reconstructive microsurgery service of the Walter Cantídio University Hospital of the Federal University of Ceará (HUWC/UFC)
Padronização técnica no treinamento em microcirurgia do serviço de cirurgia plástica e microcirurgia reconstrutiva do hospital universitário Walter Cantídio da Universidade Federal do Ceará (HUWC/UFC)
ABSTRACT
INTRODUCTION: The microsurgery technique was introduced by Jacobsen and Suarez in 1960, and is defined as the procedure applied in handling small structures with the aid of magnifying glasses. Microsurgery has expanding applications in different medical specialties such as plastic surgery and reconstructive microsurgery, and this complex procedure requires increasingly skilled surgeons. This article aims to present the standardization and technical concepts of the training carried out at the Plastic Surgery and Reconstructive Microsurgery Service of Walter Cantídio University Hospital of the Federal University of Ceará (HUWC/UFC).
METHOD: The microsurgery training consists of four phases with increasing levels of difficulty and a workload of 120 h for the first three stages.
RESULT: Plastic surgery residents at HUWC/UFC underwent the microsurgery training. All of the proposed objectives of the training were accomplished with satisfactory results.
CONCLUSION: Microsurgery training should start in reconstructive microsurgery laboratories, and the plastic surgeon should begin supervised surgical practice only after acquiring the skills required.
Keywords: Microsurgery; Training; Microvascular anastomosis; Plastic surgery.
RESUMO
INTODUÇÃO: A técnica microcirúrgica foi introduzida por Jacobsen e Suarez em 1960, é definida como procedimentos aplicados na manipulação de pequenas estruturas com o auxílio de lentes de aumento. A microcirurgia tem atuação em diferentes especialidades médicas, na cirurgia plástica a microcirurgia reconstrutiva, esta em continua expansão. Portanto, exige cada vez mais cirurgiões habilitados para este complexo procedimento. O artigo propõe a apresentar a padronização e os conceitos técnicos do treinamento realizado no serviço de cirurgia plástica e microcirurgia reconstrutiva do Hospital Universitário Walter Cantídio da Universidade Federal do Ceará.
MÉTODO: O treinamento em microcirurgia é realizado através de quatro fases com níveis crescentes de dificuldade, com uma carga horária de 120h para as três etapas iniciais.
RESULTADO: Os residentes de cirurgia plástica do HUWC/UFC foram submetidos ao treinamento em microcirurgia, tendo sido contemplado todos os objetivos propostos com resultado satisfatório.
CONCLUSÃO: O Treinamento em microcirurgia deverá ser iniciado em laboratórios de microcirurgia reconstrutiva, somente após desenvolver habilidades necessárias, o cirurgião plástico deverá iniciar a prática cirúrgica supervisionada.
Palavras-chave: Microcirurgia; Capacitação; Anastomose microvascular; Cirurgia plástica.
Microsurgical techniques are used in many specialties such as orthopedics and traumatology, plastic surgery, neurosurgery, gynecology, obstetrics, urology, organ transplant surgery, otorhinolaryngology, ophthalmology, and cardiac and thoracic surgery1,2. Microsurgery is defined as the surgical procedure applied in handling small structures that is carried out with the aid of magnifying glasses (or a microscope)1,3.
Jacobsen and Suarez, in 1960, introduced the microsurgical technique, and started a new era in reconstruction and tissue transfer procedures by using the principles of vascular sutures defined by Carrel1,3-8. In 1965, at an experimental level, Buncke introduced the use of the microscope in plastic surgery and, in 1972, he performed the first microsurgical transfer (omentum) for scalp coverage, by using instruments adapted from watchmaker's tools4,9. The first inguinal flap was successfully transplanted with a microsurgical procedure and described by Daniel in 1973. In South America, experimental vascular microsurgery was introduced in 1971 at the Microsurgical Laboratory of the Faculty of Medicine of the University of São Paulo by Ferreira, and the first microsurgical replantation successfully performed in humans was a hand replant carried out by Ferreira in 1972. The successful replantation of fingers and limbs significantly improved this area of scientific knowledge1,3,5,10.
The evolution of reconstructive microsurgery is divided into three distinct phases. In the first decade (1970s), microsurgery emerged as an additional resource to reconstructive surgery, and was reserved for situations in which conventional alternatives had already failed or could not be used. In the 1980s, i.e., the second phase, microsurgical reconstruction incorporated significant amount of concepts, anatomical knowledge, and reports referring to the high survival rate of the flaps used, thus demonstrating the safety of the technique and increasing its clinical indications. In the third phase, from 1990, microsurgical flaps were no longer considered an additional option but became the main choice in many cases6,11-18.
Reconstructive microsurgery continues to expand, and there is an increasing requirement for more trained and qualified surgeons to perform these complex procedures 1,14,15. Nowadays, microsurgery should be considered as the first-choice resource to achieve better functional and aesthetic outcomes, even when the possibility to use different and less complex methods exists 1,3,12,13,. Ideally, microsurgery training should be carried out in reconstructive microsurgery laboratories, as the surgeon should start clinical practice in the operating room only after acquiring proper skills and experience in the laboratory. The field of microsurgical procedures requires a high degree of dedication, patience, and continuous training. In most states of Brazil, there are no training centers or regular microsurgery courses, with the cost of training being one of the main obstacles. Therefore, the existence of a reconstructive microsurgery laboratory should be considered essential for a resident training program in plastic surgery1,4,5,11-13,16-20.
In 2002, the Plastic Surgery and Reconstructive Microsurgery Service of Walter Cantídio University Hospital of the Federal University of Ceará (HUWC/UFC) presented a new approach for training on vascular microanastomosis, which involved the preparation of tubes of different diameters (smaller diameter of 0.5mm) from latex gloves, in which microanastomoses were carried out16. In 2009, a proposal for an initial training program in microsurgery was developed. It consisted of three phases, in which 60h was dedicated to the first two phases. The aim of the first phase was to become familiar with the microscope and the instruments used in microsurgical practice. The second phase was centered on suture training on latex gloves. The third phase aimed to consolidate the knowledge and skills by training on the surgical technique and performing vascular sutures by using a model of animal segments 4. In 2013, the training routine in microsurgery was improved and became part of the medical residency program in plastic surgery.
OBJECTIVE
The aim of this study is to present the technical concepts and standardization of the microsurgery training undergone by plastic surgery residents at the Plastic Surgery and Reconstructive Microsurgery Service of HUWC/UFC.
METHOD
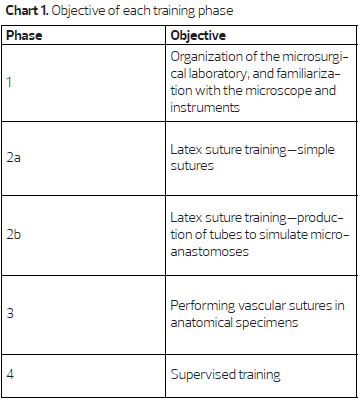
The microsurgery training at the Plastic Surgery and Reconstructive Microsurgery Service of HUWC/UFC comprised four phases with increasing levels of difficulty and carried out twice a week, 4 h per day, with a workload of 120 h for the first three phases (Chart 1).
The first training phase consists of a theoretical part and familiarization with the optical instruments (magnifying glasses and microscope) and surgical instruments used in microsurgery. The workload for this phase is 20 h. It is necessary to learn the correct positioning of the surgeon: seated comfortably at table height, with proper support for the hands, forearms, and elbows, and positioned perpendicularly to what needs to be manipulated. The chair should be adjustable. The positioning of the hands is another important factor. The instruments should be supported by the second finger while the thumb and the second finger perform the pincer movement (Figure 1). It is essential to learn how to properly handle the microscope, including all the necessary adjustments for good visualization of the operative field. Therefore, the surgeon should adjust the interpupillary distance, with maximum magnification (40'); the focus, as precisely as possible; and the system of eye diopter, to achieve the best acuity possible starting with the left eye. A microscope with a magnification of 16-40' and magnifying glasses of 3.5' and 4' were used.
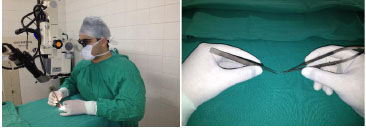
Figure 1. Positioning of the surgeon and the surgeon's hands on the operating table.
The instruments used for training in the microsurgery laboratory were as follows: straight (wire cutting) and curved scissors (dissection); straight (suture) and curved forceps (end-to-side anastomoses); single and double vascular clamps with an approximation bar; forceps to position the clamps; needle-holder; and 8-0, 9-0, 10-0, and 11-0 nylon threads (Figure 2).
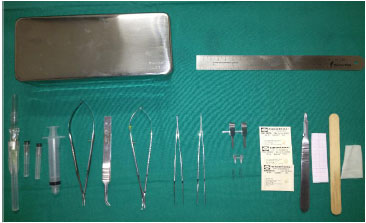
Figure 2. Surgical instruments.
The aim of the second phase of the training is to learn how to perform microsurgical sutures and microanastomoses. This phase is divided into two subphases and has a workload of 40 h. The aim of phase 2a is to learn simple sutures performed on latex glove segments. The aim of phase 2b is to simulate anastomoses by using latex tubes.
In phase 2a, the surgeon has to mount a latex glove segment on a wooden straw without keeping it too tight, secure the assembly to the worktable, perform a straight incision at different positions by using straight forceps and scissors, and perform separate sutures with three nodes each. Threads of different diameters should be used in decreasing order, starting with those needing magnifying glasses for visualization and proceeding to those needing increased magnification with the microscope. For a more precise and rigorous technique, it is necessary to avoid holding the edges of the suture with forceps or needle holder, as well as to be prepared to wear an apron, mask, cap, and surgical gloves, as if in the operating room.
In phase 2b, for the production of tubes, rubber rectangles measuring 3'1cm, obtained from the surgical gloves, were fixed to wooden straws on a graph paper of the same size. Then, two parallel incisions were made at distances that varied according to the diameter of the tube to be obtained. The two incisions were then closed with continuous or separate sutures. Therefore, for example, to create a cylinder of 1mm in diameter, the distance between the edges should be 3.14mm, as the distance corresponds to the length of the cross-section of the cylinder created and follows the mathematical formula 2Πr (where r is the radius of the circle and Π is the mathematical constant with a value of 3.14). Then, the surgeon proceeds to section the tube crosswise and carry out a simple suture to simulate the anastomosis. The initial suture is performed with three cardinal points at a distance of 120º to each other; thus, given the first point, the surgeon should proceed with the second suture in the same work plan, as the third point should be performed after a 180º clamp rotation in the center of the area in which the anastomosis is carried out. Then, the surgeon should perform the suture between each point, starting in the caudal region and finally rotating the clamp and finishing the suture in the anterior wall (Figure 3).
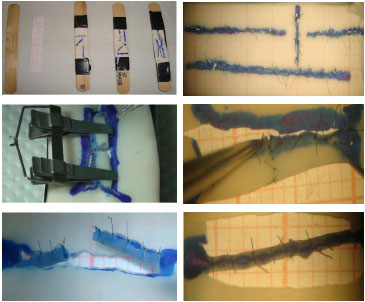
Figure 3. Assembly of the glove to the wooden straw; microsurgical sutures in different directions; and the production of latex tubes to carry out microanastomoses (phase 2).
In the third phase of the training, the surgeon learns how to perform nerve and vascular sutures by carrying out vascular suture and micro-neurorrhaphy in animal anatomical parts (poultry thigh). The aim is to establish the basis for application in humans. This phase has a workload of 60h.
The surgeon should select vessels from the poultry thigh of 0.5mm to 2mm in diameter, dissect the vessels with straight forceps and curved scissors (Figure 4), incise the vessel with a curved scissors, place the vascular clamps according to the vessel diameter, dry the adventitial layer of the vessel on which the suture will be made, and wash the vessel stumps with saline solution and heparin to remove possible residual thrombi. Then, the surgeon should perform light dilation of the vascular stumps, carry out an end-to-end suture according to the technique described in phase 2b, remove the distal and proximal clamps, catheterize the proximal stump with Jelco 24, inject the serum, and observe the patency of the suture.
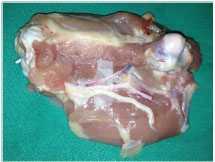
Figure 4. Nerve, artery, and vein of a dissected poultry thigh.
The surgeon should select the nerve passing alongside the great vessels in the poultry thigh, dissect the nerve with straight forceps and curved scissors, section across the nerve with curved scissors, approximate the stump with two 9.0 or 10.0 wire sutures diametrically opposed in the epineurium, leave the long wires of the surgical node to facilitate the manipulation of this nerve, complement the previous suture with two 60º distant points from those diametrically opposed, rotate the nerve to 180º by using opposite nodes as repair nodes, and suture the caudal face with two more points (Figure 5).

Figure 5. Micro-neurorrhaphy in the poultry thigh: step-by-step artery preparation, microarterial anastomosis, and vein preparation, with removal of intra-luminal clots and microvenous anastomosis. After the procedure, the vessel is catheterized with Jelco 24 and saline solution is injected under pressure to test the resistance and permeability of the suture.
The fourth phase of microsurgery training consists of continuous and supervised training. In this phase, the resident surgeon starts to practice in the operating room, becoming involved in surgeries that use free flaps for oncological or posttraumatic reconstructions and reconstruction of peripheral nerves. The supervised surgical practice is initiated for the safety of the resident, after having learnt the surgical times with an initial structures dissection with surgical magnifying glasses, preparation of microsurgical flaps and realization of micro-vascular and nervous anastomoses (Figure 6). This phase covers the 3 years of residency in plastic surgery at the Plastic and Reconstructive Microsurgery Service of HUWC/UFC.
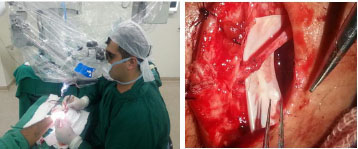
Figure 6. Supervised training (phase 4) on radial sensory nerve micro-neurorrhaphy.
RESULTS
The residents at the Plastic Surgery Service of HUWC/UFC underwent a microsurgery training that accomplished all the proposed objectives and produced satisfactory results. The phases of the training were not considered expensive and were followed by a supervised training that lasted for the entire medical residency in plastic surgery.
DISCUSSION
The microsurgical training described is long and continuous, and requires great dedication to be successful. The existence of a reconstructive microsurgery laboratory is essential for plastic surgery residents to carry out a training program in this area. Training should always be started in an environment that does not involve patients, owing to the complexity of the technique1,4,5,11,20.
The use of latex gloves is a well-established option, as it allows training on performing sutures in different directions, angles, and inclinations. In addition, it allows training on anastomosis. However, it has the disadvantage of not having a consistency similar to that of biological tissues and of not allowing to practice on dissection techniques1,4,5,16.
The use of anatomical parts is extremely important for the training and these are easy to obtain, such as the poultry thigh for instance. Moreover, they can be stored for days in coolers without losing consistency. Poultry vessels and nerves are an excellent model for training microsurgical techniques, as they present characteristics similar to those of human vessels and do not require a structured laboratory. In addition, the integrity of the anastomosis can be tested by injecting saline solution under pressure. In this phase, the resident will be able to learn how to handle and feel delicate microsurgical structures, practice their dissection, and perform vascular microanastomoses and micro-neurorrhaphy4,5,12.
Training with live animals raises increasing discussions on ethics concerning animal surgical practice. Moreover, the maintenance of an animal facility implies high additional costs to the expenses already required for the organization of a training center, related to surgical instruments, wires, and optical equipment4,5,13,16.
This model of microsurgery training performed at the Plastic Surgery and Reconstructive Microsurgery Service of HUWC/UFC has the clear advantages of reduced costs and greater ease, as it does not use animal models. After the training routine and an adequate workload for an appropriate, comfortable, and continuous period, it is possible to observe how the manual skills of the resident have evolved during the training. In this practice, the surgeon becomes gradually familiar with the existing refinements of the procedure until becoming qualified for the supervised surgical practice. The supervised practice is performed during the 3 years of medical residency, with the purpose that, at the end of the residency, the resident would be able to carry out microsurgical procedures.
CONCLUSION
The microsurgical training, with a well-defined routine, qualified supervision, and low-cost experimental materials proved to be effective and allowed the acquisition of theoretical knowledge and satisfactory skills. The routine of the microsurgery training at HUWC/UFC is in agreement with trainings carried out in other services. The consolidation of this training, now as part of the requirements for the plastic surgery resident at HUWC/UFC, aims to satisfy the constant need for more trained and qualified surgeons to perform microsurgical procedures.
REFERENCES
1. Zumiotti AV, Mattar JR, Rezende MR, et al. Manual de Microcirurgia. Instituto de ortopedia e traumatologia, Laboratório de Rotary de Microcirurgia Reconstrutiva. Ed. Atheneu 2008.
2. Ferreira LM. Guias de Medicina Ambulatorial e Hospitalar Unifesp - Escola Paulista de Medicina - Cirurgia Plástica. 1º ed. Manole, 2007. p.121-126.
3. Carreirão S. Cirurgia Plástica para a Formação do Especialista. Ed. Atheneu, 2012. p.141-158.
4. Dias IS, Pessoa SG, Macêdo, JE, et al. Treinamento inicial em microcirurgia. Rev Bras Cir Plást. 2010;25(4):595-599.
5. Lima DA, Galvão MS, Cardoso MM, et al. Rotina de treinamento laboratorial em micro cirurgia do instituto Nacional do Câncer. Rev Bras Cir Plást. 2012;27(1):141-149.
6. Mélega JM, Viterbo F, Mendes FH. Cirurgia Plástica "Os Princípios e a Atualidade". Ed. Guanabara Koogan, 2011. p.225-231.
7. Pessoa BB. Reconstrução parcial da língua com retalho microcirúrgico. Rev Bras Cir Plást. 2009;24(4):437-440.
8. Torres AL, Milcheski DA, Nakamoto HA, Junior PT, Ferreira MC. Aplicação da microcirurgia no reparo de lesões complexas. Rev Bras Cir Plást. 2009;24(2):131-137.
9. Cunha MS, Ramos RS, Torres AL, et al. Aplicação da microcirurgia no serviço de cirurgia plástica da Universidade Federal da Bahia: análise dos resultados e complicações. Rev Col Bras Cir. 2005;32(6):23.
10. Souza Filho MV, Santos CC. Microcirurgia em reconstruções complexas: análise dos resultados e complicações. Rev Bras Cir Plást. 2009;24(2):123-130.
11. Martins PN, Montero EF. Basic microsurgery training. Comments and proposal. Acta Cir Brás. 2009;22(1):2007-2079.
12. Webster R, Ely PB. Treinamento em microcirurgia vascular: É economicamente viável? Acta Cir Bras. 2002;17(3):110-115.
13. MacDonald JD. Learning to perform microvascular anastomosis. Skull Base. 2005;15(3):229-240.
14. Viterbo F. The importance of microsurgery in plastics. Rev Bras Cir Plást. 2012;27(1):2.
15. Isolan GR, Santis-isolan PM, Dobrowolski S. Considerações técnicas no treinamento de anastomoses microvasculares em laboratório de microcirurgia. J Bras Neurocirurg. 2010;21(1):8-17.
16. Pessoa BB, Pessoa SG. Treinamento em microanastomoses utilizando tubos de látex. Acta Cir Bras. 2002;17(2):143-146.
17. Pessoa BB, Pessoa SG. O Retalho hipogástrico cutâneo no cão: modelo para o aprendizado experimental de microcirurgia. Acta Cir Bras. 2002;17(3):25-33.
18. Pessoa SG, Riquet GF. Fundamentos básicos de microcirurgia vascular: estudo experimental. Ceará Méd. 1982;4(1):10-16.
19. Martins PNA, Montero EFS. Organization of a microsurgery laboratory. Acta Cir Brás. 2006;21(3):187.
20. Acland RD. Equipment for microsurgical practice. In: Acland RD, editor. Microsurgery practice manual. St. Louis: C.V. Mosby; 1980. p.7-16.
1 - General Surgeon - Resident Physician at the Plastic Surgery and Reconstructive Microsurgery Service of Walter Cantídio University Hospital of the Federal University of Ceará, Fortaleza, CE, Brazil; Aspirant Member of the Brazilian Society of Plastic Surgery (BSPS)
2 - Member of the Brazilian Society of Plastic Surgery (BSPS) - Regent of the Plastic Surgery and Reconstructive Microsurgery Service of Walter Cantídio University Hospital of the Federal University of Ceará, Fortaleza, CE, Brazil
3 - Member of the Brazilian Society of Plastic Surgery (BSPS) - Plastic Surgeon and Preceptor of the Plastic Surgery and Reconstructive Microsurgery Service of Walter Cantídio University Hospital of the Federal University of Ceará, Fortaleza, CE, Brazil
4 - Specialist Member of the Brazilian Society of Plastic Surgery (BSPS) - Plastic Surgeon and Preceptor of the Plastic Surgery and Reconstructive Microsurgery Service of Walter Cantídio University Hospital of the Federal University of Ceará, Fortaleza, CE, Brazil
5 - Professor of Medicine at the Federal University of Ceará, Fortaleza, CE, Brazil - Member of the Plastic Surgery and Reconstructive Microsurgery League Dr. Germano Riquet
Institution: Plastic Surgery and Reconstructive Microsurgery Service of Walter Cantídio University Hospital, Fortaleza, CE, Brazil.
Correspondent author:
Caio Alcobaça Marcondes
Street Arquiteto Reginaldo Rangel, N° 155, apt. 1901, Bairro Cocó
Fortaleza-CE. Brasil. - CEP: 60192-320
E-mail: caio_alcobaca@hotmail.com
Article received: January 10, 2014
Article accepted: June 1, 2014


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter