

Ideas and Innovation - Year 2014 - Volume 29 -
Techniques for removal of primary grafts from traumatic skin flaps in areas with degloving injuries
Técnicas para retirada de enxertos primários de retalhos traumáticos em áreas de desenluvamento
ABSTRACT
Primary grafting using skin from traumatic flaps is essential in the correct and early treatment of patients with degloving injuries. Split- or full-thickness grafts can be used; however, the literature does not yet provide any indication of the best option. Moreover, this skin also can also be used immediately or after tissue bank storage. This report describes the main techniques for graft removal from traumatic flaps.
Keywords: Skin transplantation; Soft tissue injuries; Wound closure techniques; Skin/Surgery; Fascia/Surgery.
RESUMO
No tratamento dos pacientes vítimas de desenluvamento atendidos de maneira correta e precoce, a enxertia primária, com utilização da pele proveniente dos retalhos traumáticos é fundamental. Podem ser utilizados enxertos em ambas as espessuras, parcial ou total, não existindo na literatura uma definição em relação à melhor opção. Esta pele, também, pode ser utilizada de maneira imediata ou após conservação em banco de tecidos. Descrevemos neste artigo as principais técnicas para retirada de enxertos dos retalhos traumáticos.
Palavras-chave: Transplante de pele; Lesões dos tecidos moles; Técnicas de fechamento de ferimentos; Pele/Cirurgia; Fáscia/Cirurgia.
Degloving injuries are characterized by avulsion of the skin and subcutaneous tissue from the muscle fascia plane, and these injuries involve lesions of fasciocutaneous perforators and musculocutaneous segmental vessels. They result from the application of sudden and high intensity forces with tangential vectors, promoting compression, stretching, twisting, and friction1-4.
The most critical point in the initial evaluation of degloved injuries is to determine blood supply and viability of the traumatized tissue, which is not always a straightforward procedure. As such, a detailed clinical evaluation by an experienced professional is essential. Considering the occurrence of lesions in the perforating vessels, the blood supply of the degloved segment depends on the dermal and subdermal plexus, capable of keeping limited length segments from the degloved area1,5,6.
In the initial evaluation and resuscitation phase, patients are usually under the care of the general surgery, pediatric surgery, and orthopedics teams. The assessment by the plastic surgeon is essential and should be performed as early as possible, preferably during the resuscitation phase in combination with assessments by other experts, facilitating therapeutic decisions1.
Specialists may make errors during primary procedures or cause delays in assessment requests. The main issue is indication of a simple resuture of traumatic flaps to the bed of origin, with a high incidence of necrosis and infection2-8.
The use of traumatized skin with and without blood supply, prepared for primary grafting (full- or split-thickness) was originally described by Farmer9,10 in 1939; this method is considered the optimal management procedure for covering skin of the affected area, with high efficiency and low morbidity.
There is still debate as to the best option regarding grafts withdrawn from traumatic flaps3,6-8,11,12. In principle, all available skin should be used, even from areas with signs of friction burns (Figure 1). If there are no appropriate conditions for the integration, this skin will still function temporarily as a biological dressing; the skin of amputated limbs may also be used1,2,13 (Figure 2).
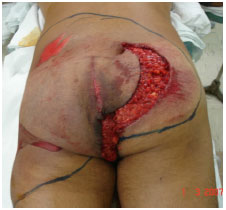
Figure 1. Case 1 - shows a hit and run victim with the buttocks being caught under moving tires. The degloved segment is marked with a surgical pen (approximately 13% of the body surface). Note the central area with riction burns
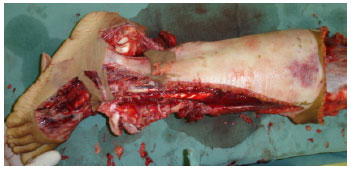
Figure 2. Case 2 - shows an amputed segment subjected to the removal of split - thickness skin grafts.
Temporary preservation of grafts
In the presence of large muscle in juries or fractures, hemodynamic instability, coagulopathy, long surgical procedures, and/or large local contamination, one can opt for the traumatic flap skin preservation at a tissue bank1-3,6.
Described by Hueston and Gunter14, this option allows for the skin to be stored for later use when the wound bed is in the best condition, that is, when it presents with less exudation and devitalized/contaminated tissue and more of the granulation tissue, in addition to the stability of critically ill patients1,3,5,6,15.
The skin is withdrawn and prepared, both in full- as well as split-thickness procedures, and can be stored under conventional refrigeration (4ºC) and used within a few days, usually with good integration indices. The skin is preserved in saline solution and antibiotics for a period of 7 to 14 days, and preferably is used within the first 2 days2-4,6,15.
Withdrawal of partial skin grafts
Both a Blair knife and an electric dermatome can be used for this procedure, with the latter allowing for the removal of skin slices that are uniform in length and thickness. For example, one can take into account the irregularities in thickness, particularly at the margins of traumatic flaps.
Skin graft removal can be done in situ, with the traumatic flap resutured7,8 or temporarily reattached3 (Figures 3 and 4). Another option is to pull using tongs, held by an assistant, for better placement. During the removal of the grafts, the dermis of degloved segments that do not present perfusion/bleeding is an optimal reference for guiding the extent of tissue resection7,8 (Figure 5).
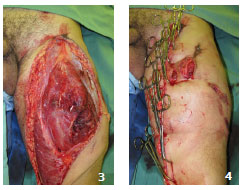
Figures 3 and 4. Case 3 - shows a degloved segment in the anterior/lateral posterior region of the left thigh (approximately 10% of the body surface). Note the preparation for withdrawal of in situ grafts and the temporary fixation with Backhaus tweezers.
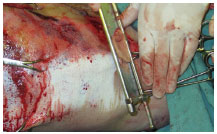
Figure 5. Case 3 - shows in situ withdrawal of split-thickness grafts with a Blair knife. Note the absence of dermal blood flow In the degloved segment.
In the ex situ option, the segment should be initially sectioned, and to reveal skin perfusion definition, a shaving test can be used, that is, initial incisions can be made to evaluate the presence of blood flow and bleeding. The removed segments are positioned (under tension, pulled using tweezers16 by an assistant17) on a supporting surface such as a kidney dish or a 1,000 mL saline flask, on an auxiliary table17 (Figures 6 and 7).The ex situ option is most appropriate in cases of polytraumatized patients who are being subjected to other priority operations (for example, general surgery, neurosurgery, or orthopedic surgery). This is also the case in patients with hemodynamic instability, in situations that hinder the efficiency of the plastic surgeon, and in situations where preparation and positioning for in situ graft removal are required.
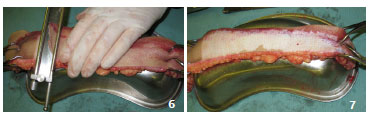
Figures 6 and 7. Case 4 - shows an ex situ withdrawal of split-thickness grafts with a Blair knife, held on an auxiliary table with a segment fixed and pulled using tweezers, supported on a kidney dish.
Partial skin graft may be indicated for very critical situations, considering the higher possibility of integration. This is the preferred procedure in the literature5,7,8,12,18,19. Unfortunately, the aesthetic results are often poorer, mainly under expansion or prior slitting (mesh graft).
Withdrawal of full-thickness skin grafts
When opting for full-thickness graft removal, the most common method is ex-situ preparation, whereby the flap must be completely degreased after sectioning, either with a blade or curved scissors2,4,6,10 (Figures 8, 9 and 10). It is also possible to remove grafts in situ with a Blair knife or suitably regulated dermatome, which is normally necessary to complement degreasing.
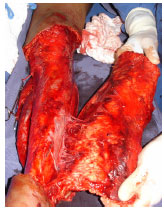
Figure 8. Case 5 - shows a circumferential degloved segment in the left leg (approximately 7% of the body surface) and resection of the traumatic flap for ex situ preparation in a hemodynamically unstable patient with abdominal trauma.
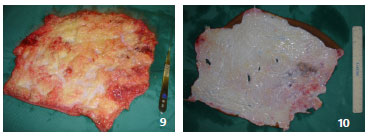
Figures 9 and 10. Case 5 - shows an ex situ full-thickness graft preparation; degreasing is performed with scissors and a razor.
Under these conditions, the standards for clinical assessment of dermal blood flow is identical compared to the aforementioned parameters. In addition, the conduct of the plastic surgeon regarding hemodynamic instability and/or priority operations is also identical to that mentioned above, in relation to the removal of partial skin grafts.
Full-thickness skin grafting entails delayed preparation, but is technically simple, and thus, suitable for bedridden receivers in better condition. These procedures have better functional and aesthetic results because of the lower secondary contracture, and are the preferred methods, as indicated in several reports 2,4,6,10,11,20,21.
CONCLUSIONS
Importantly, raw areas secondary to degloving that lack the possibility of using the traumatic flap as the donor area (primary grafting) owing to delays or incorrectly performed procedures require the removal of untraumatized skin from other donor sites. These sites may not be of sufficient size, and may require multiple surgical recovery periods and/or longer hospital stays. With these considerations in mind, we highlight the importance of initial care to degloving injuries and the different technical options for performing primary grafting.
REFERENCES
1. Mello DF, Demario LA, Solda SC, Helene Jr A. Desenluvamentos fechados - Lesão de Morel-Lavallée. Rev Bras Cir Plast. 2010;25(2):355-60.
2. Mandel MA. The management of lower extremity degloving injuries. Ann Plast Surg. 1981;6(1):64-8.
3. Kudsk KA, Sheldon GF, Walton RL. Degloving injuries of the extremity and torso. J Trauma.1981;21(10):835-9.
4. Hidalgo DA. Lower extremity avulsion injuries. Clin Plast Surg .1986;13(4):701-10.
5. Milcheski DA, Ferreira MC, Nakamoto HA, Tuma Jr P, Gemperli R. Tratamento cirúrgico de ferimentos descolantes nos membros inferiores - proposta de protocolo de atendimento. Rev Col Bras Cir. 2010;37(3):199-203.
6. Letts RM. Degloving injuries in children. J Ped Orthop.1986;6:193-7.
7. Ziv I, Zeligowsgi AA, Elyashuv O, Mosheiff R, Lilling M, Segal D. Immediate care of crush injuries and compartment syndrome with the split-thickness skin excision. Clin Orthop Rel Res.1990;256:224-7.
8. Zeligowsgi AA, Ziv I. How to harvest skin graft from the avulsed flap in degloving injuries. Ann Plast Surg.1987;19(1):85-7.
9. Farmer AW. Whole skin removal and replacement. Ann Surg. 1939;110(5):1440-7.
10. Farmer AW. Treatment of avulsed skin flaps. Ann Surg.1939;110:951-9.
11. Jeng SF, We FC. Technical refinement in the management of circumferentially avulsed skin of the leg. Plast Recons Surg.1997;100:1434-41.
12. Anderson WD, Stewart KJ, Wilson Y, Quaba AA. Skin grafts for the salvage of degloved below knee amputation stumps. Br J Plast Surg.2002;55:320-323.
13. Southern SJ, Hart NB, Venkatramakishnan V, Nieuwoudt F, Villafane O. Lower limb salvage using parts of the contralateral amputated leg. Injury.1997;28(7):477-9.
14. Hueston JT, Gunter GS. Primary cross-leg flaps. Plast Recons Surg. 1967;40(1):58-62.
15. Sheridan R, Mahe J, Walters P. Autologous skin banking. Burns. 1998;24:46-8.
16. Goris RJA, Nicolai PA. A simple method of taking skin grafts from the avulsed flap in degloving injuries. Br J Plast Surg.1982;35:58-9.
17. Dickson JK, Mills C, Devarraj V. Surgical tip: Simple technique for harvesting split thickness skin grafts from degloved skin. J Plast Reconstr Aesthet Surg.2010;63(2):e233.
18. Cohen SR, LaRossa D, Ross AJ, Christofersen M, Lau HT. A trilaminar skin coverage technique for treatment of severe degloving injuries of extremities and torso. Plast Recons Surg.1990;86(4):780-784.
19. Kottmeier SA, Wilson SC, Born CT, Hanks GA Iannacone WM. Surgical management of soft tissue lesions associated with pelvic ring injury. Clin Orthop Rel Res.1996;329:46-53.
20. Gibson T, Ross DS. Dermatome for preparing large skin-grafts from detached skin and fat. Lancet. 1965;1(7379):252-3.
21. DeFranzo AJ, Marks MW, Argenta LC, Genecov DG. Vaccum assisted closure for the treatment of degloving injuries. Plast Recons Surg.1999;104(7):2145-8.
1 - Master in Surgery - Plastic and Cranio-Maxillo-Facial Surgeon. Assistant Physician at the Plastic Surgery Service of the Irmandade da Santa Casa de Misericórdia de São Paulo (ISCMSP)
2 - Plastic Surgeon - Assistant Physician at the Plastic Surgery Service of ISCMSP
3 - PhD in Surgery - Head of the Plastic Surgery Service of ISCMSP
Institution: Irmandade da Santa Casa de Misericórdia de São Paulo - ISCMSP
Corresponding author:
Daniel Francisco Mello
Rua Nanuque, 335, Apto 84 - Vila Leopoldina
05302-031 - São Paulo/SP
E-mail: mello.plastica@gmail.com
Article received: January 18, 2013
Article accepted: April 13, 2013


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter