

Original Article - Year 2013 - Volume 28 - Issue 4
Evaluation of topic linoleic acid application on random-pattern skin flaps in rats
Avaliação da aplicação tópica de ácido linoleico em retalhos cutâneos randomizados em ratos
ABSTRACT
BACKGROUND: Concern about the viability of skin flaps, especially those of random pattern, has been the subject of several studies in plastic surgery. The limiting factor of these flaps is the unpredictability of its microvasculature, which could lead to partial or complete necrosis of the flap. This study tested the role of topical use of linoleic acid on the survival of random skin flaps. This is a polyunsaturated fatty acid, which in wound healing studies showed to act by mediating the inflammatory response, and decreasing the production of nitric oxide, accelerating the repair process.
OBJECTIVE: The aim of this project was to evaluate morphometric and histologically the improvement on skin flaps survival in rats treated with topical linoleic acid.
METHOD: 24 male Wistar rats randomly divided into 3 groups, the control group or Glycerin (GG, n = 8), Dersani® group (GD, n = 8) and Linoleic Acid group (GL, n = 8). Under general anesthesia, a cranial base modified McFarlane random skin flap was elevated in dorsal region of the rats.
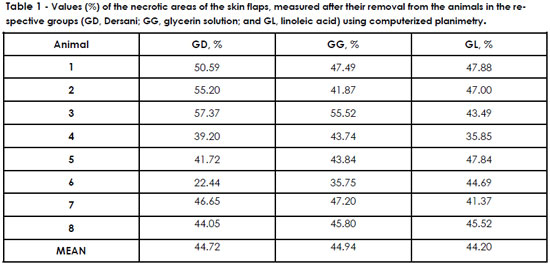
RESULTS: The mean percentage of necrosis area among the groups was: GG - 44.49%, GD - GL and 44.72% - 44.20%. It showed no statistically significant differences (p> 0.05). Histological analysis has also not found differences in the degree of necrosis or inflammation.
CONCLUSION: In this study, topical application of LA showed no statistically significant differences (P>0.05) on improving the survival area of random skin flap in rats.
Keywords: Rats. Ischemia. Reperfusion. Surgical Flaps. Linoleic Acid.
RESUMO
INTRODUÇÃO: A preocupação com a viabilidade de retalhos cutâneos, especialmente os randomizados, tem sido motivo de diversas pesquisas na cirurgia plástica. O fator limitante para a utilização desses retalhos é a imprevisibilidade de sua vascularização distal, o que poderá resultar em necrose parcial ou completa do retalho. Neste estudo, foi testado o uso tópico do ácido linoleico na melhoria da sobrevivência dos retalhos de pele. Este é um ácido graxo poli-insaturado, que em estudos sobre cicatrização de feridas mostrou atuar na mediação da resposta inflamatória e na diminuição da produção de óxido nítrico, acelerando o processo de reparo.
OBJETIVO O objetivo deste trabalho foi avaliar morfométrica e histologicamente o aumento da viabilidade de retalhos cutâneos randomizados em ratos, tratados com uso tópico do ácido linoleico.
MÉTODO: Vinte e quatro ratos Wistar machos divididos aleatoriamente em três grupos: grupo Controle ou Glicerina (GG, n = 8), grupo Dersani® (GD, n = 8) e grupo Ácido Linoleico (GL, n = 8). Sob anestesia geral, foi elevado um retalho randômico modificado de McFarlane na região dorsal dos ratos.
RESULTADOS: A média da porcentagem da área de necrose dos retalhos foi: GG - 44,49%, GD - 44,72% e GL - 44,20%. Estas não apresentaram diferenças estatísticas significativas (P>0,05). A análise histológica não evidenciou diferenças significativas no grau de necrose ou processo inflamatório.
CONCLUSÃO: Neste estudo, a aplicação tópica do AL não apresentou diferenças estatísticas significativas em aumentar a área de viabilidade do retalho cutâneo randomizado em ratos.
Palavras-chave: Rato. Isquemia. Reperfusão. Retalhos cirúrgicos. Ácido Linoleico.
The improvements in plastic surgery techniques has allowed the reconstruction of extensive defects secondary to trauma, genetic abnormalities, and tumor removal, especially with the use of skin flaps. The distal vasculature of the random skin flap is unpredictable, and its viability depends on microcirculation. The extent of its blood supply is determined during its construction; the use of an inappropriate area, which exceeds the bed supplied by the feeding vessels, or damage to its vascular integrity may lead to partial or complete tissue necrosis1.
Necrosis can lead to dehiscence, infection, and delayed wound healing. Furthermore, additional reconstructive procedures (debridement, resuture, and grafting) are usually required, albeit with low efficiency cosmetic or functional results, leading to prolonged hospital stays, greater number of outpatient visits, and increased treatment costs2.
In 1967, Myers and Cherry3 described the technique of skin flap delay as an effective means to increase its survival. First, an incision and suture of the flap area are made, and after a minimum period of 7 days, transfer is performed. A procedure with 2 surgical sessions is involved. Since then, improvement of the distal survival of random flaps and replacement of the delay procedure remains a challenge in plastic surgery and has stimulated the implementation and improvement of clinical and experimental studies4.
A preocupação com a viabilidade dos retalhos cutâneos tem sido motivo de pesquisas na cirurgia plástica. Entre as estratégias de pesquisas, existem diferentes abordagens usando agentes físicos inespecíficos locais, agentes farmacológicos e fatores do crescimento, no intuito de promover maior viabilidade dos retalhos cutâneos5. The viability of skin flaps has been the subject of research in plastic surgery. Among the research strategies being implemented, different approaches using local nonspecific physical agents, pharmacological agents, and growth factors to promote greater viability of skin flaps have been applied5.
After the creation of a skin flap, blood vessels and sympathetic nerves become damaged during the incision of the flap and the dissection of the original bed for transfer, which results in a hemodynamic imbalance with decreased blood flow and tissue oxygenation, creating a progressive ischemia gradient toward its distal margins. Inadequate blood perfusion and ischemia/reperfusion (I/ R) are considered the most important risk factors of flap necrosis. As the nutritional needs of the skin are relatively low, a large number of vascular territories can be recruited before necrosis occurs6.
In one study, an essential fatty acid used topically (i.e., oleic acid) led to improved survival of skin flaps subjected to I/R in experimental models7. Meanwhile, linoleic acid, an unsaturated fatty acid, is easy to obtain and inexpensive, and is present in high concentrations in oilseeds (sunflower oil, canola, and flaxseed). Studies on wound healing showed that it works in mediating the inflammatory response and decreases nitric oxide production, thus contributing to the acceleration of wound healing8. Linoleic acid plays an important role in the transport of fats, in the maintenance of the integrity and function of cell membranes, and as a local immunogen. Although widely used in the care of different types of wounds, including surgical wounds, the benefit of using essential fatty acids (EFA), especially in commercial formulations, still lacks studies that scientifically support their applications.
Experimental studies in rats have shown that the delayed flap preparation, during which neovascularization occurs, takes approximately 7 days. Thus, in this initial period, the flap vascularization is dependent on the pedicle vessels. The formation of hematoma and edema, and even the relationship between the pedicle and flap area, can cause necrosis of the distal portion thereof. Taking into account that the skin flap is subject to ischemic phenomena that lead to the appearance of reactive oxygen species (ROS), a substance that removes these radicals can be extremely valuable at this stage. The substance should be available in the operative site in pharmacological levels and during adequate time to perform the desired action9.
Loss of flaps occurs primarily owing to hypoxia, which may be due to a change in the microcirculation, venous stasis, or poor perfusion of the distal portion of the flap. This is due to I/R injury, a common binomial mechanism, which is a consequence of biochemical events in which ROS are responsible for cellular damage.
Several pharmacological agents have been investigated for their efficacy in the prevention or reversal of ischemia in skin flaps. Some of the reportedly beneficial drugs for flap survival are anticoagulants, vasodilators, glucocorticoids, free radical scavengers, sympathomimetics, prostaglandin inhibitors, calcium channel blockers, and hemorrheologic agents10. Some studies show that the use of blocking agents that act by diverse mechanisms to block the formation of ROS, such as glutathione, dimethyl sulfoxide, and N-acetylcysteine11, are capable of minimizing I/R injuries sustained by skin flaps.
Thus, the present study aimed to examine the improvement of the viability of random skin flaps when subjected to topical treatment with an essential fatty acid, particularly linoleic acid.
METHODS
Ethical Aspects
The research project was approved by the Ethics Committee on Animal Use of the Federal University of Rio Grande (CEUA-FURG), project No. 23116.003840/2011-93 and opinion P018/2011. All the procedures strictly followed the existing regulations on animal experimentation of the Brazilian College of Animal Experimentation (COBEA).
Study Sample
Twenty-four male Wistar rats (Rattus norvegicus albinus) weighing between 350 and 400 g were randomly divided into 3 groups of 8 animals, thus respecting the principles of bioethics in research on animal experimentation. Prior to the experiment, the animals received water and commercial chow ad libitum. The light/dark cycle was set at 12 by 12 h and automatically controlled. The environmental temperature ranged from 22ºC to 25ºC.
The rats were grouped according to the different topical treatments applied in the postoperative period as follows: the control or glycerin group (GG), comparative or Dersani group (treated with a commercial mix of fatty acids and vitamins (GD), and experimental or linoleic acid group (treated with a linoleic acid solution; GL).
Anesthetic Procedure
The animals were weighed and then immediately anesthetized with a combination of xylazine chlorhydrate (Anasedan, Sespo Indústria e Comércio Ltda, Jacarei, SP, Brazil) at a dose of 25 mg/kg of weight and ketamine chlorhydrate (Dopalen, Sespo Indústria e Comércio Ltda) at a dose of 50 mg/kg of weight, administered intramuscularly in the lateral posterior right limb12. Animals were considered anesthetized after the loss of the corneal-palpebral reflex and absence of withdrawal reflex to painful stimuli applied by gripping the hind paw.
Operating Procedure
The animal was restrained on the surgical table in the prone position, and epilation of the dorsum and antisepsis with chlorhexidine 4% was performed. A flap was marked on its back through a predesigned mold (3 ' 9 cm). The base of the flap was cranial, with a distance of 1 cm below the lower edge of the scapula as the anatomical limit (Figure 1).

Figure 1 - Flap incised on the animal's dorsum, over previous markings.
The preset skin flap, consisting of superficial fascia, panniculus carnosus, subcutaneous tissue, and skin, was made using a No. 15 scalpel blade, dissected, and displaced from the underlying muscle-aponeurotic plane via blunt dissection. It was elevated from the bed, repositioned, and sutured in its original position by simple interrupted sutures using needled monofilament nylon 4.0.
Application of Solutions
Soon after the procedure, gauze soaked in 5 mL of the substances assigned to each group was applied on the flaps and maintained for 5 min. The animals remained under observation until recovery from anesthesia, assessed by the return of abolished reflexes and by active and/or stimulated movement in the cage. Over the next 6 days, repeated application of gauze soaked in the respective solutions was performed for the same period (5 min), once a day, without the need for anesthesia or sedation of the animals, as these were provided by the habituation of the animals to their handlers and a peaceful environment.
Procedure for Collection of Materials and Euthanasia
On the seventh day, the animals were euthanized in a CO2 chamber, as recommended by the American Veterinary Medical Association specifically for animal experiments. The flaps were removed and photographed using a digital camera (Sony HX 100) at a focal length of 20 cm, with the presence of a millimeter ruler for reference. The photographs were then analyzed with the aid of the AxioVision 40 V 4.6.3 program (Carl Zeiss Imaging Solutions, GmbH, Germany), with demarcation of the total area of the flaps and flap necrosis, and subsequent calculation of the percentage of necrosis, using the computerized planimetric technique (Figure 2).

Figure 2 - Flap removed from the animal to be subjected to computerized planimetry. Measurement values of the total area (red) and necrotic area (blue).
The percentage of necrosis of each flap was calculated7 by dividing the necrotic area by the total area of the flap, multiplied by 100. For histopathological analysis, a distal fragment (3 ' 1 cm from the distal end of the flap) and medial fragment (3 ' 1 cm, obtained in the area between 4.5 and 5.5 cm of the flap) were removed (Figure 3).

Figure 3 - Distal and medial fragments removed from the flap for histopathological analysis.
After euthanasia on day 7 and removal of tissue samples necessary for the study, the animals were prepared, frozen, and discarded according to the requirements of ethical principles for experimental studies of the COBEA (Sao Paulo, Brazil, 1991).
Preparation of Solutions
Linoleic acid 99% (Sigma-Aldrich, St. Louis, MO, USA) diluted in ethanol stock solution was prepared at 30 µM in a solution of glycerol and 0.02 M Tris-HCl at pH 7.4 (1:1 by volume). Solutions were prepared daily at the time of use to prevent oxidation13,14.
The glycerol group received the same vehicle as the linoleic acid group but without linoleic acid. In the comparison group, the Dersani commercial mix (Saniplan, Rio de Janeiro, RJ, BR) was used, with the following composition: triglycerides of capric and caprylic acids, clarified sunflower oil, lecithin, retinyl palmitate, tocopheryl acetate, and alpha-tocopherol.
Histological Study
The sections were placed on slides and stained with hematoxylin-eosin for analysis. Microscopic examination was performed on a Zeiss Axioskop 40 microscope (Zeiss, Oberkohen, Germany). Photographs were taken with a CoolSnap Pro micro camera (Media Cybernetics, Bethesda, USA).
The slides were examined under light microscopy by a pathologist blinded to the grouping of the rats. For this study, 2 sections were selected per slide, avoiding artifacts due to traction, folds, or other changes that would misrepresent the assessment.
Two criteria were evaluated, namely the presence of necrosis and inflammation. The percentage areas of inflammation and necrosis were evaluated with an image analysis system using the Image Pro Plus 4.5.1 program (Media Cybernetics, Bethesda, USA), with the planimetry method based on random digital photographs and counting at least 20 fields with a 2,400 mm2 grid at '100, according to the methodology proposed by Gundersen et al.15.
Statistical Analysis
All the data were expressed as mean (SD). To analyze the results, the tests were applied according to the nature of the variables.
The percentage of necrotic area was compared between the 3 groups (GG, GD, and GL) using the analysis of variance (ANOVA), with 5% significance (□). The comparison of the means (Tukey test, unprotected) was also applied. The same tests were applied to assess the degree of necrosis of the distal and medial fragments of each group, which were analyzed histopathologically.
All the data were analyzed using the Statistica 7.0 software.
RESULTS
No loss of animals undergoing the procedure and no complications such as infection, autophagy, or suture dehiscence were observed.
The means of the necrotic areas as measured by computerized planimetry were 44.72% in the GD, 44.94% in the GG, and 44.20% in the GL, presented in detail in Table 1. No statistically significant differences between the means were observed (ANOVA level of significance [□] of 5%; graphic 1).

Graphic 1- Mean percentages of necrosis in the distal fragments of the flaps in the different groups, evaluated histologically (GGD, glycerin; GDD, Dersani; and GLD, linoleic acid). No statistically significant differences were observed between the means (ANOVA, level of significance [a] of 5%).
The mean percentages of necrosis in the distal fragments removed from the flaps were 91.66% for GG, 64.16% for GD, and 56% for GL (graphic 2). The mean percentages of the degree of necrosis in the medial fragments removed from the flaps were 51.66% for GG, 50.83% for GD, and 44.16% for GL (graphic 3).

graphic 2 - Mean values of necrosis (%) in the distal fragments of the flaps in the different groups, evaluated histologically (GGD, glycerin; GDD, Dersani; and GLD, linoleic acid). No statistically significant differences were observed between the means (ANOVA level of significance [a] of 5%).

graphic 3 . Mean values of necrosis (%) in the medial fragments of the flaps in the different groups, evaluated histologically (GGM, glycerin; GDM, Dersani; and GLM, linoleic acid).No statistically significant differences were observed between the means (ANOVA level of significance [a] of 5%).
DISCUSSION
The present study used the random cranial base skin flap proposed by McFarlane et al.16, in 1965, as this is traditionally the most used experimental model to study necrosis and its avoidance. The rat was used as the experimental model because of ease in obtaining, handling and accommodation, and resistance to manipulation, surgeries, and infectious processes. Moreover, the animal is an internationally accepted experimental model. No cases of local irritation or allergies caused by substances under study were observed.
According to our results, topical application of a linoleic acid solution at a concentration of 30 µM, according to the method proposed by Cardoso et al.8 (2004), repeated once a day, did not modify the percentage area of necrosis (%) as assessed by planimetry (P = 0.05), compared with the commercial compound of fatty acids and vitamins used in the control or comparative group.
When we analyzed the histopathological changes of injury due to I/R in the medial and distal fragments removed from the skin flap, the degree of necrosis observed in the linoleic acid group, although not statistically significant (p = 0.05), presented a trend toward a lower degree of injury to the distal fragments (Figure 2). Owing to the small sample size, this trend may not have shown a statistically significant difference (P = 0.05).
When performing a random skin flap, which is a common procedure, ischemia and necrosis are relatively common and dreaded problems. Any intervention that may improve the viability of the flap warrants scientific and technological advances. Different treatments have been studied to achieve this goal.
The delayed flap preparation technique, in which an ischemic preconditioning of the flap is performed, is effective in improving the viability of the flap and is often used for this purpose. However, it has the disadvantage of being a 2-step procedure17.
The use of pharmacological agents in the prevention or reversal of ischemia in skin flaps has been widely studied. However, systemic application in relatively high doses of the pharmacological agents that have demonstrated benefits is generally needed, though with greater possibility of systemic adverse effects18.
The topical application of agents on the flaps to minimize the incidence of necrosis of its distal portion may represent a significant reduction in the occurrence of undesired potential systemic side effects19. This strategy has been proven as effective in some studies. Hsu et al.7, in 2004, used oleic acid via adjuvant penetration and found that it significantly increased the viable area of flaps.
Livaoglu et al.18, in 2010, demonstrated that the topical use of Hirudoid (mucopolysaccharide polyphosphate) decreased the necrotic area of flaps. Huemer et al. 19, in 2003, reviewed the application of an ointment used as an analgesic, especially by sportsmen (Finalgon), which consisted of 2 angiogenic substances, namely Nicoboxil and Nonivamide, which increase blood flow to tissues when administered topically. They found a statistically significant reduction in the area of necrosis and suggest that such a treatment could have a prophylactic application to skin grafts at risk.
No published studies were found on the application of linoleic acid on skin flaps. However, in studies on induced healing of skin wounds, linoleic acid accelerated healing when compared with linolenic and oleic acids and strongly inhibited the local production of nitric oxide8. Rojo et al.20, in 2010, also observed a higher healing rate of induced skin wounds in rats and transgenic zebrafish, in addition to an increase in angiogenesis. However, in the present study, when analyzed from a morphological perspective, no significant differences were observed when compared with the other substances under study and with known antioxidant properties.
In some studies, an increased number of daily applications were performed, with intervals of 6 to 8 h. This may be a factor in the effectiveness of the treatment19. In most studies, however, the application was performed once a day with very mixed results.
CONCLUSION
In this study, the topical administration of linoleic acid did not reduce significantly the necrotic area of the random skin flaps in the rats. Concerning the degree of necrosis, no statistically significant difference was observed between the groups.
The commercial mix of fatty acids evaluated (Dersani) showed no greater benefit than the other substances assessed.
REFERENCES
1. da Rocha FP, Fagundes DJ, Pires JA, da Rocha FS. Effects of hyperbaric oxygen and N-acetylcysteine in survival of random pattern skin flaps in rats. Indian J Plast Surg. 2012;45(3):453-8.
2. Vural E, Key JM. Complications, salvage, and enhancement of local flaps in facial reconstruction. Otolaryngol Clin North Am. 2001;34(4):739-51.
3. Myers MB, Cherry G. Augmentation of tissue survival by delay: an experimental study in rabbits. Plast Reconstr Surg. 1967;39(4):397-401.
4. Holm C, Mayr M, Höfter E, Becker A, Pfeiffer UJ, Mühlbauer W. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg. 2002;55(8):635-44.
5. Reichenberger MA, Keil H, Mueller W, Herold-Mende C, Gebhard MM, Germann G, et al. Comparison of extracorporal shock wave pretreatment to classic surgical delay in a random pattern skin flap model. Plast Reconstr Surg. 2011;127(5):1830-7.
6. Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63(4-5):300-11.
7. Hsu OK, Gabr E, Steward E, Chen H, Kobayashi MR, Calvert JW, et al. Pharmacologic enhancement of rat skin flap survival with topical oleic acid. Plast Reconstr Surg. 2004;113(7):2048-54.
8. Cardoso CR, Souza MA, Ferro EA, Favoreto S Jr, Pena JD. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004;12(2):235-43.
9. Almeida KG, Fagundes DJ, Bochetti Manna MC, Montero EFS. Ação do dimetil-sulfóxido na isquemia de retalhos randômicos de pele em ratos. Acta Cir Bras. 2004;19(6): 649-57.
10. Frangoulis M, Georgiou P, Chrisostomidis C, Perrea D, Dontas I, Kavantzas N, et al. Rat epigastric flap survival and VEGF expression after local copper application. Plast Reconstr Surg. 2007;119(3):837-43.
11. Yoshida WB, Campos EBP. Isquemia e reperfusão de retalhos cutâneos: efeitos do manitol e vitamina C na redução de áreas de necrose em modelo experimental no rato. Acta Cir Bras. 2005;20(5):358-63.
12. Massone F. Anestesiologia veterinária: farmacologia e técnicas. 5a ed. Rio de Janeiro: Guanabara Koogan; 2008.
13. Pereira LM, Hatanaka E, Martins EF, Oliveira F, Liberti EA, Farsky SH, et al. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem Funct. 2008;26(2):197-204.
14. Cardoso CR, Favoreto S Jr, Oliveira LL, Vancim JO, Barban GB, Ferraz DB, et al. Oleic acid modulation of the immune response in wound healing: a new approach for skin repair. Immunobiology. 2011;216(3):409-15.
15. Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96(5):379-94.
16. Mcfarlane RM, Deyoung G, Henry RA. The design of a pedicle flap in the rat to study necrosis and its prevention. Plast Reconstr Surg. 1965;35:177-82.
17. Morris SF, Taylor GI. The time sequence of the delay phenomenon: when is a surgical delay effective? An experimental study. Plast Reconstr Surg. 1995;95(3):526-33.
18. Livaoğlu M, Kerimoğlu S, Sönmez B, Livaoğlu A, Karaçal N. The effect of Hirudoid on random skin-flap survival in rats. J Plast Reconstr Aesthet Surg. 2010;63(6):1047-51.
19. Huemer GM, Wechselberger G, Otto-Schoeller A, Gurunluoglu R, Piza-Katzer H, Schoeller T. Improved dorsal random-pattern skin flap survival in rats with a topically applied combination of nonivamide and nicoboxil. Plast Reconstr Surg. 2003;111(3):1207-11.
20. Rojo LE, Villano CM, Joseph G, Schmidt B, Shulaev V, Shuman JL, et al. Wound-healing properties of nut oil from Pouteria lucuma. J Cosmet Dermatol. 2010;9(3):185-95.
1. Postgraduate and Administrative Technician
2. PhD, Associate Professor of Anatomy, Federal University of Rio Grande, Rio Grande, RS, Brazil
3. PhD, Associate Professor of Pathology, Pontifical Catholic University of Rio Grande do Sul (PUC-RS), Head of the Service of Pathology and Cytopathology, Hospital São Lucas, PUC-RS, Porto Alegre, RS, Brazil
4. PhD, Assistant Professor, Institute of Mathematics, Statistics and Physics Federal University of Rio Grande, Rio Grande, RS, Brazil
5. MSc, PhD student, postgraduate program in Health Sciences, Federal University of Rio Grande, Rio Grande, RS, Brazil
6. Medical student, Federal University of Rio Grande, Rio Grande, RS, Brazil
7. Medical student, Federal University of Rio Grande, Rio Grande, RS, Brazil
Marcelo Luís Altenhofen da Silva
Rua Dr. Nascimento, 737 Centro
Rio Grande - RS. CEP: 96200-380
E-mail: marcelo.altsilva@gmail.com
Article received: 9/10/2013
Article accepted 23/11/2013
Work performed as a master dissertation, Graduate Program in Health Sciences, Federal University of Rio Grande, Rio Grande, RS,Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter