

Case Report - Year 2013 - Volume 28 -
Pyoderma gangrenosum after reduction mammaplasty: case report and discussion
Pioderma gangrenoso pós-mamoplastia redutora: relato de caso e discussão
ABSTRACT
We report the case of a 31-year-old woman who underwent mammoplasty and abdominoplasty and developed a rare and recurrent skin disease, of unknown etiology that was diagnosed as pyoderma gangrenosum. While the diagnosis was clinical, the treatment was empirical. An association between this disease and other systemic diseases was observed, in addition to pathergy (development of a new inflammatory lesion in areas of trauma) and changes in cellular immunity. The patient was empirically treated with antibiotics, sulfone, corticosteroids, and topical agents and experienced complete wound healing with no recurrence.
Keywords: Pyoderma gangrenosum. Rare skin disease. Immunosuppression.
RESUMO
Neste artigo relata-se o caso de uma jovem de 31 anos de idade, submetida a mamoplastia e abdominoplastia, que evoluiu com uma doença cutânea rara, recorrente e de causa desconhecida: pioderma gangrenoso. O diagnóstico é clínico e o tratamento permanece empírico. Tem-se encontrado relação entre essa afecção e outras doenças sistêmicas, além de evidências do fenômeno de patergia (desenvolvimento de uma nova lesão inflamatória na área do trauma) e de alterações da imunidade celular. A paciente foi tratada empiricamente com antibióticos, sulfona e corticoide, e com tratamento tópico, evoluindo para o fechamento completo das lesões sem apresentar recorrência.
Palavras-chave: Pioderma gangrenoso. Doença cutânea rara. Imunossupressão.
Pyoderma gangrenosum is a rare, recurrent, and destructive skin disease of unknown etiology1-10, mainly affecting women aged between 25 and 55 years1,3. The differential diagnosis was based on the condition of the patient1-4,8. Here, we report the case of a female patient who developed post-reductive mammoplasty pyoderma gangrenosum.
CASE REPORT
A 31-year-old female patient underwent reductive mammoplasty combined with abdominoplasty as part of a treatment strategy. She was discharged 24 h after surgery; however, she complained of pain in the inguinal region radiating to the right lower limb.
Owing to persistent abdominal pain 72 h after the first surgery, we decided to perform a second surgical intervention of abdominoplasty during which we noticed a right inguinal-abdominal nerve plication and absence of local signs of infection. A few hours after the second intervention, the patient developed fever (body temperature 38ºC) and significant local edema, with extravasation of serosanguineous secretion (seroma) that infiltrated the subcutaneous cellular tissue and reached the breasts. Formation of local bullae with subsequent ulceration, elevated margins, and no purulent secretion were observed. Three days after the second surgery, the same cutaneous lesions appeared in approximately 75% of the left breast and 50% of the right breast.
Prophylactic treatment with antibiotics was already started during the first surgery and administration of cephalexin was continued as part of the therapeutic treatment regimen for 3 days after the first surgery. Then, ciprofloxacin was administered in combination with ceftriaxone for 5 days, but the patient showed no signs of improvement.
The patient was suspected to have pyoderma gangrenosum; therefore, 10 mg of dexamethasone was administered between 6 and 8 days after the second surgery, after which we hospitalized the patient. We requested the dermatology department of the hospital to confirm pyoderma gangrenosum. Thereafter, the patient was administered prednisone (20 mg) and diaminodiphenyl sulfone (50 mg) for 15 days. Two sample secretions were collected and cultured, and both yielded negative results. Moreover, on the suggestion of the infectious diseases department, empirical treatment was started with vancomycin and amikacin.
The topical treatment included saline, essential fatty acids, cellulose acetate impregnated with an emulsion of petrolatum, and gauze dressing twice a day. The patient was also administered 10 sessions of therapy with hyperbaric chamber.
The patient was discharged 15 days later and was prescribed prednisone and diaminodiphenyl sulfone for 30 days, with gradually tapered dosage and 10 additional hyperbaric chamber therapy sessions. The patient showed satisfactory response to treatment and complete wound healing.
Figures 1 to 4 illustrate the case reported.
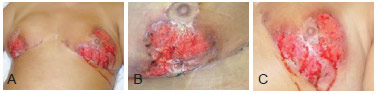
Figure 1 - Images of the breasts 15 days after the beginning of the treatment. In A, frontal view of both breasts. In B, image of the right breast. In C, image of the left breast.
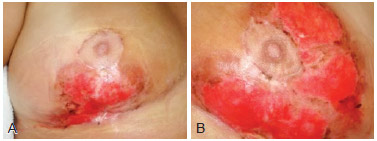
Figure 2 - Images of the breasts 25 days after beginning treatment. In A, image of the right breast. In B, image of the left breast.
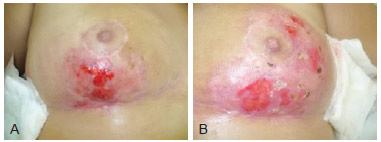
Figure 3 - Images of the breasts 55 days after beginning treatment. In A, image of the right breast. In B, image of the left breast.
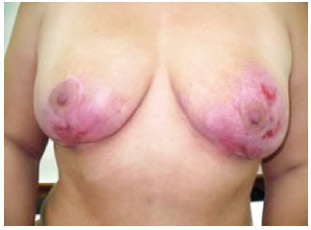
Figure 4 - Image of the breasts 104 days after beginning treatment (frontal view).
DISCUSSION
Pyoderma gangrenosum was initially described by Brocq and Simon, in 1908, as geometric fagedenism, and the term pyoderma gangrenosum was later coined by Brunsting et al. in 19309.
It is a disease of unknown etiology, characterized by the presence of hemorrhagic pustules, painful nodules, or single/multiple vesicopustules that are well defined and of variable sizes. These lesions evolve into destructive ulcers that expand concentrically and present well-defined margins, of which the external drops sharply to the bottom of the affected area, and the external margins merge with the normal surrounding skin, accompanied with edematous infiltration and slightly elevated and violaceous papules, with a necrotic core containing blood, pus, and granulation tissue; the bottom of the affected area is traversed by microabscesses that resemble tunnels and caves1-3,8,9. Of note, the typical lesion of pyoderma typically starts as a sterile pustule with a necrotic core surrounded by red-bluish tissue that quickly evolves into a fast-growing ulcer, but with negative culture results9. The trigger lesions of the patient described in this report were multiple vesicles that spread rapidly. Peaks of fever are associated with this condition, although no bacterial growth is observed in the cultures.
Pyoderma is idiopathic in 40-50% of the cases, and in 50% of the cases, it develops secondary to a systemic disease; 10% of all cases are misdiagnosed.
Several diseases should be included in the differential diagnosis (Table 1), namely ulcers induced by vasculitis triggered by antiphospholipid antibody in lupus, venous insufficiency, polyarteritis nodosa, Wegener's granulomatosis, skin lesions associated with malignant tumors such as leukemia and lymphoma, sporotrichosis, herpes simplex type 2, tuberculosis, cutaneous amoebiasis, Münchausen syndrome, leishmaniasis, cellulitis, mycoses, skin lymphoma, post-operatory gangrene, and syphilis2-4,7,8.
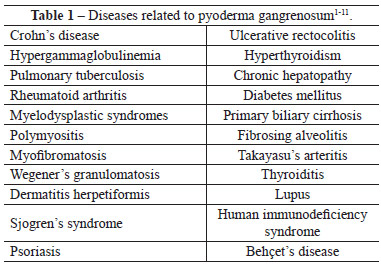
The diseases commonly associated with pyoderma confirm the theory of altered immune response1,9, especially as cases of pyoderma triggered by sclerosis of varicose veins (predominantly in the lower limbs), vaccines, local trauma, debridements, grafts, and surgical scars have been reported 2-6.
The diagnosis is clinical and the treatment remains empirical1,10. An association between pyoderma and systemic diseases, pathergy (development of new inflammatory lesions in the areas of trauma), and changes in cellular immunity have been observed2,4,6,10. It is important to emphasize that the patient developed this condition after a second surgical intervention, thus confirming this association. Initially, small pustules appeared, which later developed into extremely painful, fast-growing concentric ulcers with irregular and elevated margins after breakage. The ulcers were edematous and infiltrated by granulation tissue in their inferior limit and resembled the ulcers described in previously reported cases4-6.
The findings mentioned above are not specific or diagnostic laboratory findings1,2,10. However, several associations with autoimmunity have been noticed in this patient, such as thrombocytosis and hypergammaglobulinemia type A. Due to a variable inflammatory infiltrate, the biopsy is unspecific1,9, and therefore, it was not performed in the case reported.
The progression time of pyoderma is variable and can range from 15 days to 10 years1. It can occur after any type of trauma9 - in our patient's case, the trauma was caused by the surgery itself. Fever and significant toxicity commonly occur in the acute phase of pyoderma. Our patient experienced peaks of fever hours before the onset of this disease1,6.
Owing to the unknown etiology of pyoderma, the treatment is quite variable and, in most cases, consists of immunosuppressants1,2,6. For local treatment, the use of the following drugs has been reported: potassium permanganate, iodopovidone, silver nitrate, hexachlorophene, topical rifampicin, gentian violet, intralesional triamcinolone, benzyl peroxide, sodium cromoglycate, 0.9% saline, hydrocolloids, calcium alginate, and hyperbaric chamber. After undergoing 10 sessions of hyperbaric chamber therapy, the patient showed satisfactory to complete wound healing2,3,5,6,8.
Systemic treatment consisted of prednisone (40 g/d to 60 g/d), alone or in combination with clofazimine at a dose of 100 mg/d or even sulfasalazine, sulfasalazine, sulfone, azithromycin, cyclophosphamide, mycophenolate mofetil, thalidomide, rifampicin, gammaglobulin, plasmapheresis, minocycline, infliximab, tacrolimus, and methotrexate1-3,5,6,8,9,11. Based on the experience of the dermatologist following this case, we decided to treat the patient with prednisone and diaminodiphenyl sulfone.
At present, in the tenth month of follow-up, the patient shows no signs of recurrence. Complete wound healing was observed approximately 4 months after the onset of the symptoms.
Pyoderma gangrenosum is a rare skin disease whose timely diagnosis is extremely important because if treatment is delayed, the patient can experience serious health consequences. Although there is no consensus regarding the universal treatment of this condition, the use of corticosteroids and/or immunosuppressants should be seen as the first step to prevent the progression of these lesions into ulcers.
REFERENCES
1. Wanke NCF, Souza MAA, Régnier GC, Maceira J. Pioderma gangrenoso: a respeito de dez casos. An Bras Dermatol. 1994;69(3):175-8.
2. Sanchez TG, Schneider G, Pereira MC, Azevedo LEL, Azevedo GL, Souza MM, et al. Pioderma gangrenoso: relato de caso. Comun Ciênc Saúde. 2006;17(3):237-41.
3. Fraga JCS, Souza VL, Valverde RV, Gamonal A. Pioderma gangrenoso: apresentação atípica. An Bras Dermatol. 2006;81(5 Supl 3):S305-8.
4. Batista MD, Fernandes RL, Rocha MAD, Ikino JK, Pinheiro RF, Chauffaille MLLF, et al. Pioderma gangrenoso bolhoso e síndrome mielodisplásica. An Bras Dermatol. 2006;81(5 Supl 3):S309-12.
5. Campbell G, Campbell I, Leite RMS, Batista MS. Uso da clofazimina no pioderma gangrenoso: a propósito de um caso. An Bras Dermatol. 1993;68(5):287-90.
6. Souza CS, Chiossi MPV, Takada MH, Foss NT, Roselino AMF. Pioderma gangrenoso: casuística e revisão de aspectos clínico-laboratoriais e terapêuticos. An Bras Dermatol. 1999;74(5):465-72.
7. Burkieviecz CJC, Schmidt L, Silva MB, Skare TL. Pioderma gangrenoso e lúpus eritematoso sistêmico. Rev Bras Reumatol. 2007;47(4):296-9.
8. Hevia H, Suárez J, Vergara MT. [Peri-ileostomic pyoderma gangrenosum. Report of one case]. [Article in Spanish] Rev Med Chil. 2004;132:747-9.
9. Pires AMKS, Weiss PB, Weissbluth ML, Ramos MC, Hampe SV, Bakos L. Pioderma gangrenoso associado a hepatite cônica ativa. An Bras Dermatol. 1987;62(1):15-8.
10. Cabral VLR, Miszputen SJ, Catapani WR. Anticorpo anticitoplasma de neutrófilos (ANCA) em pioderma gangrenoso, um marcador sorológico para associação com doenças sistêmicas: estudo de oito casos. An Bras Dermatol. 2004;79(1):39-44.
11. Costa IMC, Nogueira LSC. Pioderma gangrenoso e artrite reumatoide - relato de caso. An Bras Dermatol. 2005;80(1):81-2.
1. Physician, specialist member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery - SBCP), Plastic Surgery Service Prof. Dr. Oswaldo de Castro, São Paulo, SP, Brazil
2. Physician, associate member of the SBCP, Plastic Surgery Service Prof. Dr. Oswaldo de Castro, São Paulo, SP, Brazil
3. Physician, member or the Sociedade Brasileira de Dermatologia (Brazilian Society of Dermatology), Plastic Surgery Service Prof. Dr. Oswaldo de Castro, São Paulo, SP, Brazil
4. Physician, associate member of the SBCP, Plastic Surgery Service Prof. Dr. Oswaldo de Castro, São Paulo, SP, Brazil
5. Physician, specialist member of the SBCP, Plastic Surgery Service Prof. Dr. Oswaldo de Castro, São Paulo, SP, Brazil
6. Physuician, pediatric surgeon, Plastic Surgery Service Prof. Dr. Oswaldo de Castro, São Paulo, SP, Brazil
Correspondence to:
Dayana Garcia Alves
Rua Vergueiro, 1.353 - 20º andar
São Paulo, SP, Brazil - CEP 04101-000
E-mail: dayana@dayanagarciaalves.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: November 19, 2010
Article accepted: May 3, 2011
This study was performed at the Serviço de Cirurgia Plástica Prof. Dr. Oswaldo de Castro (Plastic Surgery Service Prof. Dr. Oswaldo de Castro), São Paulo, SP, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter