

Original Article - Year 2013 - Volume 28 -
Adipose tissue grafting at unusual sites
Enxertos de tecido adiposo em locais pouco habituais
ABSTRACT
BACKGROUND: Body contouring has recently assumed an important role in body aesthetics. Liposuction in combination with free transplantation of adipose tissue is critical in such procedures. This study assessed the results of fat grafts implanted in unusual sites.
METHOD: Over the past 21 years, the authors have performed 4,405 adipose tissue grafting procedures; 1,407 were classified as transfers to unusual sites. The technique used is based on histological studies and is thoroughly described, including obtaining, preparation, implantation, and postoperative care. The viability of the transplanted tissue was assessed by photographic documentation, a patient questionnaire, and clinical assessment.
RESULTS: Part of the transplanted tissue was viable in 100% of cases. The percent volume that remained in the implanted site was approximately 40% of the grafted tissue. The possibility of a late increase in transplanted adipose tissue volume was considered; such changes may be associated with a lack of genetic control in the mesenchymal stem cells present in the adipose tissue.
CONCLUSIONS: Free transplants of adipose tissue in this series exhibited clinical and percent-wise progress in terms of viability, similar to grafting performed at other sites. A viability of approximately 40% of grafted adipose tissue was possible because of the care taken throughout the procedure.
Keywords: Autogenous fat grafting. Fat grafting. Fat tissue grafting in low habitual sites.
RESUMO
INTRODUÇÃO: Recentemente a definição do contorno corporal assumiu importante papel na estética do corpo. A lipoaspiração associada aos transplantes livres de tecido adiposo é fundamental nesses procedimentos. O objetivo deste estudo foi avaliar os resultados dos enxertos gordurosos implantados em locais pouco habituais.
MÉTODO: Nos últimos 21 anos, os autores realizaram 4.405 enxertos de tecido adiposo, dos quais 1.407 foram classificados como transferidos para locais pouco habituais. A técnica utilizada é fundamentada em estudos histológicos e descrita em todas as suas etapas, isto é, na obtenção, no preparo, na implantação e nos cuidados pós-operatórios. A viabilidade do tecido transplantado foi avaliada através de documentação fotográfica, de questionário respondido pelos pacientes e de avaliação clínica.
RESULTADOS: Foi possível comprovar a viabilidade de parte do tecido transplantado em 100% dos casos. O porcentual do volume que permaneceu no local implantado foi de aproximadamente 40% do tecido enxertado. Os autores chamam a atenção para a possibilidade tardia de aumento do volume do tecido adiposo transplantado, e relacionam essa alteração como um provável descontrole gênico das células-tronco mesenquimais presentes no tecido adiposo.
CONCLUSÕES: Os transplantes livres de tecido adiposo avaliados nesta série apresentaram evolução clínica e porcentual de viabilidade semelhantes aos de enxertos realizados em outros locais. A viabilidade em torno de 40% do tecido adiposo enxertado foi possível graças aos cuidados tomados em todas as etapas do procedimento, conforme a técnica descrita.
Palavras-chave: Enxerto de gordura autóloga. Enxerto de gordura. Enxerto de tecido adiposo em sítios pouco habituais.
Ever since Ilhouz1 reported the use of liposuctioned adipose tissue for grafting, variants of each step of the original technique have been introduced, including retrieval, preparation, and implantation of the adipose tissue in the recipient bed as well as postoperative care. Despite the difficulty of the late assessment of the percentage of viable tissue as a result of using the various techniques, all of them result in long-term viability of at least part of the transplanted tissue2-9.
Histologic studies about fat grafting in rodents and humans demonstrate 3 important points: partial viability of transplanted tissues; quantitative variations in volume and percentage of viable tissue related to technical aspects of fat collection, preparation and implantation; and when fat grafting is adequately executed the viable remaining volume is about 40-50%10-18.
Fat grafting has been used to fill depressions in facial rejuvenation and body contouring. However, they are seldom used in some anatomical regions such as the trochanter, lips, perioral, areola, sternal furcula, mandibular contour, chest, vulva, nose, and thighs. Thus, this study presents our experience with fat grafting in those regions.
METHOD
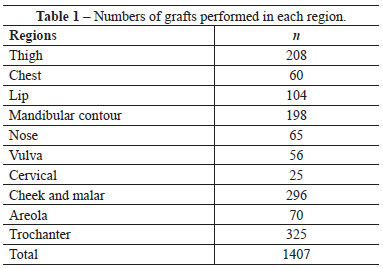
Over the last 21 years, we treated 2,315 patients with a total of 4,405 adipose tissue grafts. In 932 patients (including 831 women and 101 men), 1,407 transplantations were performed in regions classified as uncommon (Table 1). The patients were between 9 and 72 years old. The grafted volumes in a single session were between 2 and 1,200 mL; the same region was grafted up to 5 times. One female patient was transplanted with a total of 3,100 mL over 4 sessions.
Smaller volumes of adipose tissue were obtained through liposuction using 60-mL syringes attached to a cannula 3 mm in diameter and 15 cm long with 3 distal non-sequential holes. For larger volumes, a liposuction machine was used; adipose tissue was collected in an intermediate 500-mL sterile bottle. The vacuum of the liposuction machine was set to a maximum of 500 mmHg, and during the procedure, the aspirated tissue was transferred to an auxiliary bottle every 3 to 5 min, thereby reducing the low-pressure exposure of the tissue in the collection bottle. In such cases, cannulas 20 or 25 cm long and 4 mm in diameter with 3 distal non-sequential holes were used.
Prior to liposuction, 0.9% saline was introduced both in the syringe and collection bottle to 10-15% of the container's volume. No detachment was made in the donor or recipient region, and no solution was injected, except in 8% of the patients in whom the procedures were performed with local anesthesia. In those cases, the regions were infiltrated with 0.4% xylocaine.
The time between collection and grafting was kept as short as possible. When more than 15 min had elapsed, the liquid inside the storage bottle was changed, rejecting the bloody precipitate and replenishing the volume with 0.9% saline. Following collection, the liposuctioned material was rinsed using a similar procedure until it was macroscopically blood-free.
The grafts were implanted in the recipient bed in the shape of threads or planes with an attempted maximum diameter of 4 mm. For grafts of up to 15 mL, a 20-mL syringe attached to a 5-cm-long needle with a 2-mm lumen was used. For larger volume grafts, a 60-mL syringe attached to a cannula 15 or 20 cm long with a 3-mm lumen and a single laterodistal hole was used.
No previous detachments were performed for the implantation of fatty fragments in the recipient bed. The cannula or needle was introduced in the target region; as they were pulled out, the adipose tissue was gently injected, so that the fatty fragments filled the tunnels to maintain a maximum graft diameter of 4 mm.
Whenever possible, the dressing was kept in place with a compression belt for at least 2 weeks, antibiotherapy was administered for 3 days, and NSAIDs were administered for 5 days.
Volume enhancement was postoperatively evaluated between 3 and 5 months after surgery. The evaluation was performed according 3 points: surgeon's perspective, a comparison of pre and postoperative images and by the patients using a questionnaire, including questions about volume improvement after 3 months and comparison of results after 1 week and after 3 months.
RESULTS
Of the 932 patients treated with liposuctioned adipose tissue grafts in unusual regions, the viability of transplants in the various anatomical regions was assessed. Clinical observations and photographic documentation indicated that volume gain in the grafted region was approximately 40% of the transplanted amount (Figures 1-14). However, viability rates were as low as 20% in elderly patients and those who had large amounts of free fat during the liposuction procedure for obtaining the adipose tissue. Consequently, percentuals of viability in the 72 patients were not included in the statistical evaluation of all 810 patients.
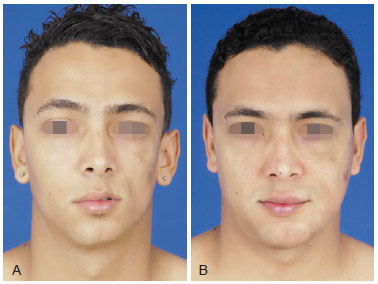
Figure 1 - A, pre-operative appearance. B, 6 months post-operative appearance after facial hemiatrophy treatment with 135 ml of fat grafting in 3 sessions.
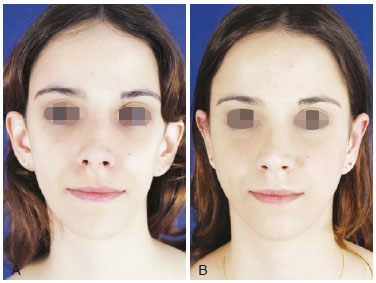
Figure 2 - A, pre-operative appearance. B, post-operative appearance with better definition of inferior lower thirds. Graft of 120 ml in just one session.
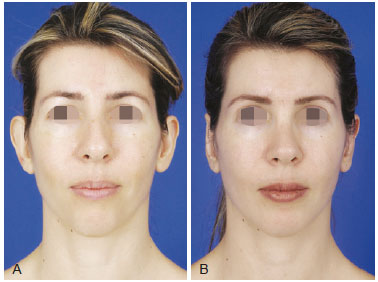
Figure 3 - A, pre-operative appearance. B, 4 months post-operative appearance. Volume enhancement of malar and mentual regions with 20 ml and 12 ml of grafts.
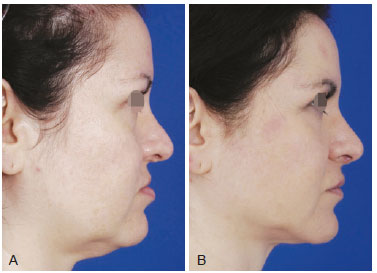
Figure 4 - A, pre-operative appearance. B, 12 months post-operative appearance. Fat grafting in 6 sessions with 6 ml in dorsal and lateral nasal areas, 15 ml for upper lip and 12 ml in lower lip depressors.

Figure 5 - A and C, pre-operative appearance. B and D, 6 months post-operative appearance. There was an increase in upper lip volume, projecting it in antero-inferior direction with 9 ml of graft in just one session.
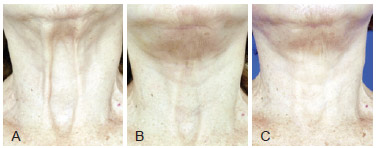
Figure 6 - A, pre-operative appearance of sternal furcula and interplatismal space depression. B, post-operative appearance after face lifting with platismal plicature and 40 ml of fat grafting. C, 3 months post-operative appearance after second session with injection of 50 ml in the same region.
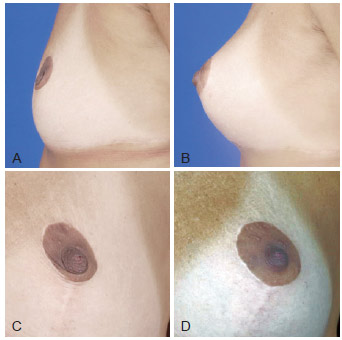
Figure 7 - A and C, pre-operative appearance. B and D, 3 months post-operative appearance. Volume enhancement of areolar region with 16 ml in 2 sessions.
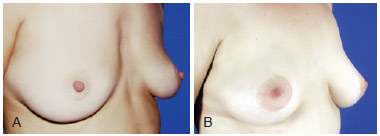
Figure 8 - A, pre-operative appearance. B, 4 years post-operative appearance. Volume enhancement of presternal region with 310 ml fat grafting in 3 sessions.
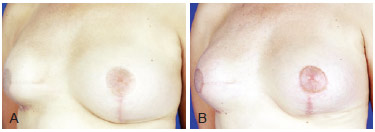
Figure 9 - A, pre-operative appearance. B, 6 months post-operative appearance. Volume enhancement of right breast to improve implant covering with 220 ml of fat grafting in 2 sessions.
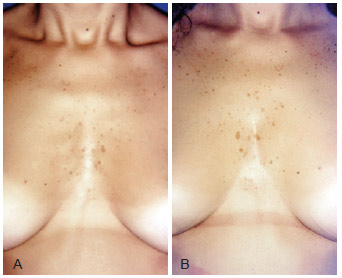
Figure 10 - A, pre-operative appearance. B, 9 months post-operative appearance. Reduction of costal arches definition with 300 ml of fat.
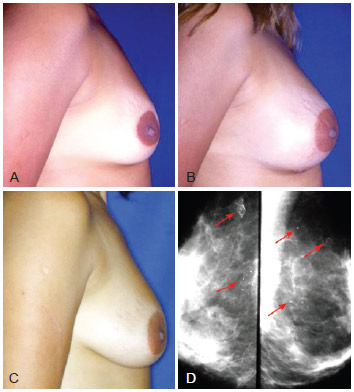
Figure 11 - A, pre-operative appearance. B, 20 days post-operative appearance of fat grafting with 225 ml in each breast in 2 sessions. C, post-operative appearance after 2 years with volume enhancement and breast ptosis. D, mamogram after 2 years with small pseudocysts and punctiform calcifications (arrows).
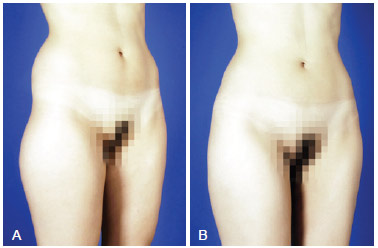
Figure 12 - A, pre-operative appearance. B, 6 months post-operative appearance. Trocanteric region definition with 270 ml on right side and 250 ml on left side.
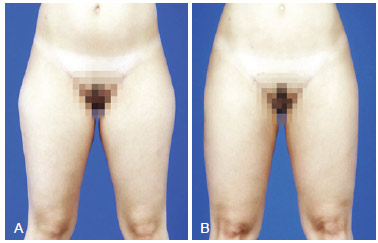
Figure 13 - A, pre-operative appearance. B, 5 months post-operative appearance. Increase in volume of inferior half of the tight with 120 ml in the lateral region and 100 ml in inner part of each limb.

Figure 14 - A, pre-operative appearance. B, 5 months post-operative appearance. Nymphoplasty with labia minora reduction and labia majora augmentation with 35 ml of fat grafts in each side.
No acute complications or infections were observed. On the other hand, late changes occurred, which were considered adverse outcomes. There were 3 cases of telangiectasia in the nasal dorsal region, 2 hyperchromatic spots on the inner-thigh region, 1 calcification, and 2 striae in the trochanter region. The most significant change in the results was a late volume increase in the transplanted adipose tissue. In some patients, this was evident from the fourth month but became better defined after the sixth month; this increase was noted in 2 trochanter grafts and 154 (23%) facial grafts (Figure 15).

Figure 15 - A, pre-operative appearance of a patient with a marked nasolabial fold. B, 2 months post-operative appearance, with 120 ml of grafts in each side in 4 sessions. C, 18 months post-operative appearance, showing a small increase of graft volume. D, 14 years post-operative appearance with marked increase in graft volume.
DISCUSSION
A viability rate of liposuctioned adipose tissue grafts of approximately 40% is within the expected range according to that reported in histological studies when grafting is performed in threads or planes with a maximum diameter of 4 mm16-18. The viability in the present study was attributable to the fact that the utmost care was taken to protect the adipose cells present in the liposuctioned adipose tissue throughout the procedure18.
Adverse outcomes may be explained by changes in the anatomy and physiology of both the transplanted adipose tissue as well as the tissues surrounding the recipient bed.
In the microenvironment of the adipose graft, vascular changes occur during resolution, which are a direct consequence of the need to increase nutrient supply and eliminate waste. This results in vascular hypertrophy and neoformation in both the recipient bed and transplanted tissue19. As such, when the graft is implanted under the skin and its peripheral region is in close contact with the vascular plexus, hypertrophy of those vessels occurs. Such an increase may lead to telangiectasias in the lower extremities, venous congestion, and melanocyte stimulation, resulting in the development of hyperpigmented skin spots. The striae observed in the trochanter region from the third month postoperatively were probably triggered by the distension of the region due to the graft's volume; this distension, associated with the natural dermal collagen balance, leads to the separation of skin fibers17,18.
Punctiform calcifications visualized on radiological images and small pseudocysts appearing in late phases of breast grafts are explained because the amount of fat grafted was greater than the capacity of the donor area to allow plasmatic embebition of the entire graft. Absence of embebition terminates into fat necrosis, explaining these late findings (Figure 11).
The late increase in the volume of the viable portion of the transplanted adipose tissue is assumed to be a consequence of adipose cell hypertrophy, especially when transplantations are performed in young people or patients experiencing weight gain20,21. In the present study, besides the causes listed above, volume increases may have also occurred as a consequence of gene activation in both transplanted mature adipocytes and mesenchymal stem cells17,18. When induced to differentiate during the inflammatory process in grafting, some undifferentiated cells differentiate into pre-adipocytes and subsequently become mature adipocytes. In that process, new fat cells are formed in the transplanted tissue, i.e., through adipogenesis. This process probably begins in the early days after grafting, i.e., during the stage of nutrition by plasma imbibition, when mesenchymal stem cells are recruited in the inflammatory process of the transplanted fat during transplantation resolution16,22-25.
In the adipose tissue, fat cells are present on a stroma of collagen and elastic fibers. Studies of this extracellular matrix have revealed the presence of mesenchymal stem cells26,28. Under certain conditions in vitro, these mesenchymal stem cells can multiply, differentiate into pre-adipocytes, and subsequently become mature adipocytes41-43. The transfer of such pre-adipocytes into living tissues allows the observation of not only the long-term residence of these cells, but also their maturation into mature adipocytes27-30.
Certain gene groups are activated during the proliferation, differentiation, and maturation of these cells; this is dependent on physical and chemical inducing factors such as hormones, growth factors, cytokines, morphology, and extracellular matrix proteins. Such communicating agents activate genes via signals that may occur intracellularly, intercellularly, or in the external environment. As such, genetic functions may be interrupted through inhibitory stimuli46-49. Several reports indicate the importance of the extracellular matrix in the induction and inhibition of genes during adipogenesis30-32.
In transplanted adipose tissue resolution, 3 factors participate in the genetic activation of mature adipocytes and/or mesenchymal stem cells during adipogenesis. The first is a physical factor resulting from the loss of anchorage by some cells; this can be observed at 2 times: during the first days, when the adipose fragments are separated and imbibed in an inflammatory exudate, and later during reabsorption of the nonviable portions of the transplanted tissue16-18,33. The second factor occurs as a result of the presence of signaling molecules of systemic and local origin present in the inflammatory exudate19-26. The third factor occurs as a result of the changes in the extracellular matrix with significant variations in the concentration, organization, and balance of collagen in the graft's microenvironment33. These agents are some of the factors responsible not only for adipogenesis in fat grafting, but also for the genetic lack of control of newly formed adipocytes or even adipocytes that have survived transplantation.
Increases in transplanted tissue volume possibly due to adipogenesis and/or gene activation in newly formed adipocytes or mature adipocytes that have survived transplantation were observed at 2 time points: (1) from the fourth month, becoming more visible from the sixth month until approximately 1 year post-grafting, and (2) after 1 year. The volume increase in the first year may reach 20% of the viable volume and is possibly related to mesenchymal stem cells that have been recruited and induced to change into pre-adipocytes during the inflammatory process in graft resolution. These cells, which develop into mature adipocytes during the first year, are mainly responsible for the volume increase of the graft.
The second increase in transplanted tissue volume occurs years later. It is slower and may increase the volume of the graft's viable adipose tissue by >100% (Figure 15). This may be due to a genetic lack of control of some mesenchymal stem cells or transplanted mature adipocytes that have been activated during the initial inflammatory stage or during transplantation resolution. These cells may have a different gene expression profile from those of normal adipocytes and/or those that have not received inhibitory stimuli. As such, after the inflammatory process is complete, these cells maintain some of their functions over several years, resulting in a late increase in graft volume.
Of the 1,407 free transplantations of adipose tissue performed in this series, 781 were followed up for 18 months; 401 of them were facial grafts, and an increase in transplanted tissue volume was observed in 154. However, some late volume increases likely occurred in all grafts.
In the present series, volume increases were more noticeable on the face, given the anatomical details of that region. Twelve patients who received bilateral grafting to the face exhibited bilateral volume increases, although they were non-uniform (Figure 15). This indicates that there are intrinsic factors in the inflammatory process during graft resolution that can lead to various late volume increases, even between the left and right sides of a patient.
CONCLUSIONS
Adipose tissue transplanted into unusual regions has a viability of approximately 40% owing to the care taken throughout the procedure. Photographic documentation and clinical outcomes indicate that this percentage has similar results among all described regions. The graft volume increases during the first year were easily noticeable on the face; late increases were observed in 154 and 22 patients who had surgery on the face and were followed up for more than 3 and 10 years, respectively.
Twenty patients with regions exhibiting graft volume increases that significantly altered facial contours were treated with liposuction, which restored shape and volume in that region.
As such, despite volume increases in adipose tissue grafts, they are presently the best option for body contouring.
REFERENCES
1. Illouz YG. L'avenir de la réutilisation de la graisse après liposuccion. Rev Chir Esthét Lang. 1984;36:13-4.
2. Bircol M, Novack BH. Autologous fat transplantation employing liposuction techniques. Ann Plast Surg. 1987;18:327-9.
3. Coleman SR. The technique of periorbital lipoinfiltration. Oper Tech Plast Reconstr Surg. 1994;1:120-6.
4. Ersek RA. Transplantation of purified autologous fat: a 3-year follow-up is disappointing. Plast Reconstr Surg. 1991;87:219-28.
5. Aboudib JHC, Cardoso CC, Gradel J. Hand rejuvenescence by fat filling. Aesthetic Plast Surg. 1992;28:559-64.
6. Colic MM. Lip and perioral enhancement by direct intramuscular fat autografting. Aesthetic Plast Surg. 1999;23:36-40.
7. Bersou Júnior A. Lipoenxertia: técnica expansiva. Rev Bras Cir Plást. 2008;23(2):89-97.
8. Cervelli C, Palla L, Pascali M, Angelis B, Curcio BC, Gentile P. Autologous platelet rich plasma mixed with purified fat graft in aesthetic plastic surgery. Aesthetic Plast Surg. 2009;33:716-21.
9. Pereira LH, Radwanski HN. Fat grafting of the buttocks and lower limbs. Aesthetic Plast Surg. 1996;20:409-16.
10. Kaufman MR, Bradley JP, Dickinson B, Heller JB, Wasson K, Hara CO, et al. Autologous fat transfer national consensus survey: trends in techniques for harvest preparation and application and perception of short- and long-term results. Plast Reconstr Surg. 1996;119:323-31.
11. Atik B, Ozturk G, Erdogan E, Tan O. Comparison of techniques for long-term storage of fat grafts: an experimental study. Plast Reconstr Surg. 2006;118:1533-7.
12. Niechajev I, Sevcuk O. Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg. 1994;9:496-506.
13. Nguyen A, Pasyk J, Bouvier TN, Hassett CA, Argenta LC. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg. 1990;85:378-89.
14. Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19:421-5.
15. Har-Shai Y, Lindenbaum ES, Lazarovich AG, Beach D, Hirshowitz B. An integrated approach for increasing the survival of autologous fat grafts in the treatment of contour defects. Plast Reconstr Surg. 1999;104:945-54.
16. Carpaneda CA, Ribeiro MT. Study of the histologic alterations and viability of the adipose graft in humans. Aesthetic Plast Surg. 1993;17:43-7.
17. Carpaneda CA, Carpaneda EM. Cirurgia Plástica Reconstructiva y Estética. Bogotá: Amolca; 2006. p. 486-7.
18. Carpaneda CA, Carpaneda EM. Manuseio de tecido adiposo em cirurgia estética. In: Melega JM, Viterbo F, Mendes FH, eds. Cirurgia plástica: os princípios e a atualidade. Rio de Janeiro: Guanabara Koogan; 2011. p. 996-1004.
19. Rossati B. Revascularisation and phagocytosis in free fat autografts: an experimental study. Br J Plast Surg. 1960;13:35-41.
20. Miller JJ, Popp JC. Fat hypertrophy after autologous fat transfer. Ophthal Plast Reconstr Surg. 2002;18:228-31.
21. Langenskiold A, Osterman K, Valle M. Growth of fat grafts after operation for partial bone growth arrest: demonstration by computed tomography scannning. J Pediatr Orthop. 1987;7:389-94.
22. Djian P, Roncari DA, Hollenberg CH. Adipocyte precursor clones vary in capacity for differentiation. Metabolism. 1985;34(9):880-3.
23. Schoeller T, Lille S, Wechselberger G, Otto A, Mowlawi A, Piza-Katzer H. Histomorphologic and volumetric analysis of implanted autologous preadipocyte cultures suspended in fibrin glue: a potential new source for tissue augmentation. Aesthetic Plast Surg. 2001;25:57-63.
24. Van RL, Roncari DA. Complete differentiation in vivo of implanted cultured adipocyte precursors from adult rats. Cell Tissue Res. 1982;225:557-66.
25. Gregoire FM, Smas SM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783-809.
26. Hausman GJ, Wright JT, Richardson RL. The influence of extracellular matrix substrata on preadipocyte development in serum-free cultures of stromal-vascular cells. J Anim Sci. 1996;74:2117-28.
27. Ibrahimi A, Bonino F, Bardon S, Ailhaud G, Dani C. Essential role of collagens for terminal differentiation of preadipocytes. Biochem Biophys Res Commun. 1992;187(3):1314-22.
28. Jones P, Schmidhauser L, Bissel MJ. Regulation of gene expression and cell function by extracellular matrix. Crit Rev Eukaryot Gene Expr. 1992;3:137-54.
29. Mandrup S, Lane MD. Regulating adipogenesis. J Biol Chem. 1997;272(9):5367-70.
30. Richardson RL, Campoin DR, Hausman GJ. Adhesion, proliferation, and adipogenesis in primary rat cell cultures: effects of collagenous substrata, fibronectin, and serum. Cell Tissue Res. 1988;251:123-8.
31. Rodríguez Fernández JL, Ben-Ze'ev A. Regulation of fibronectin, integrin and cytoskeleton expression in differentiating adipocytes: inhibition by extracellular matrix and polylysine. Differentiation. 1989;42:65-74.
32. Varzaneh FE, Shillabeer G, Wong KL, Lau DC. Extracellular matrix components secreted by microvascular endothelial cells stimulate preadipocyte differentiation in vitro. Metabolism. 1994;43:906-12.
33. Carpaneda CA. Collagen alterations in adipose autografts. Aesthetic Plast Surg. 1994;18:11-5.
1. Plastic Surgeon, Associate Member of Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery - SBCP), Surgeon of Instituto de Cirurgia do Lago (ICL), Brasília, DF, Brazil
2. Plastic Surgeon, Masters in Immunology and Applied Genetics, Universidade de Brasília (University of Brasilia - UnB), Doctor in Dermatology (UnB), Full Member of SBCP, Member of International Society of Esthetic Surgery, Medical Director of ICL, Brasília, DF, Brazil
Correspondence to:
Erick de Melo Carpaneda
ILC QI 09 - Bloco E - Térreo - Lago Sul
Brasília, DF, Brazil
E-mail: carpaneda@gmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: 23/2/2013
Article accepted: 5/10/2013
Study conducted at Instituto de Cirurgia do Lago, Brasília, DF, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter