

Original Article - Year 2013 - Volume 28 -
Comparative analysis of endoscopic transaxillary augmentation mammaplasty without endoscopic assistance
Análise comparativa entre as técnicas de mamoplastia de aumento transaxilar sem o uso de videoendoscopia e videoassistida
ABSTRACT
BACKGROUND: Transaxillary augmentation mammaplasty is a surgical option for patients who do not want scarring on the breasts. The transaxillary approach has some advantages including a lack of scarring on the breasts, maintenance of the breast parenchyma, and unaffected lactiferous sinuses. Some authors state that this procedure must be performed with endoscopic assistance in order to increase the predictability of the results. Therefore, this study evaluated the transaxillary technique with and without endoscopic assistance.
METHOD: Twenty female patients were selected and placed into 2 groups of 10 patients: those who underwent transaxillary augmentation mammaplasty with or without endoscopic assistance. Criteria including operative time, complications, postoperative pain, patient satisfaction, and esthetic parameters were assessed and compared between groups.
RESULTS: There were no significant differences between groups with respect to any criteria; complications, postoperative pain, patient satisfaction, loss of sensitivity in the breasts and papillary-areolar complex, and esthetic parameters were very similar between groups.
CONCLUSIONS: There are no postoperative differences between transaxillary augmentation mammaplasty with or without endoscopic assistance.
Keywords: Mammaplasty. Breast/surgery. Video-assisted surgery.
RESUMO
INTRODUÇÃO: A mamoplastia de aumento por via axilar é uma opção cirúrgica para pacientes que não desejam ter cicatriz na mama. A via axilar apresenta como vantagens a ausência de cicatrizes na mama, além de manutenção do parênquima mamário e ductos lactíferos inviolados. Alguns autores advogam que esse procedimento deve ser feito com o auxílio de videoendoscopia, no intuito de aumentar a previsibilidade dos resultados. O objetivo deste estudo é comparar a técnica transaxilar com descolamento sem auxílio endoscópico à técnica videoassistida.
MÉTODO: Vinte pacientes do gênero feminino foram selecionadas e alocadas em dois grupos de 10 pacientes cada. O grupo 1 foi submetido a mamoplastia de aumento transaxilar sem o auxílio de videoendoscopia e o grupo 2, a técnica videoassistida. Alguns critérios foram avaliados e comparados entre os grupos, como tempo operatório, índice de complicações, escala de dor pós-operatória, escala de satisfação das pacientes, além de parâmetros estéticos.
RESULTADOS: Não houve diferença estatística entre os grupos em nenhum dos critérios avaliados. O índice de complicações, a escala de dor no pós-operatório, o grau de satisfação das pacientes, a perda de sensibilidade nas mamas e no complexo areolopapilar (CAP), além de parâmetros estéticos mostraram-se muito semelhantes entre os dois grupos.
CONCLUSÕES: Não há diferença nos parâmetros pós-operatórios avaliados entre as técnicas de mamoplastia de aumento transaxilar sem o auxílio de videoendoscopia e a videoassistida.
Palavras-chave: Mamoplastia. Mama/cirurgia. Cirurgia videoassistida.
Transaxillary augmentation mammaplasty is a surgical option for patients who do not want scarring on the breasts. First described by Hoehler1 in 1973, the transaxillary approach has some advantages including a lack of scarring on the breasts, maintenance of the breast parenchyma, and unaffected lactiferous sinuses. Since it was first reported, some modifications as well as periareolar and inframammary techniques have been developed in order to make the technique safe and reproducible while conferring a high degree of patient satisfaction2,3. However, the conventional approach without endoscopic assistance is associated with poor and often asymmetric implant positioning, contour deformities, inadequate expansion of the lower pole, and an unpredictable appearance of the submammary sulcus4-6. Some authors state that the transaxillary technique without endoscopic assistance7,8 enables better visualization of the anatomical parameters; this provides better hemostatic control, decreases the risk of hematoma, and allows better positioning of the inframammary sulcus, producing more predictable results. In contrast, other authors state that this procedure should not be performed without endoscopic assistance9,10. Therefore, this study compared the transaxillary technique with and without endoscopic assistance.
METHOD
This study enrolled 20 female patients who underwent transaxillary augmentation mammaplasty between June 2011 and January 2012. The inclusion criteria were as follows: age between 20 and 40 years, absence of comorbidities, breast hypoplasia, no regular medication use except for contraceptives, and no mammary ptosis11. Patients with a family history of breast cancer, previous breast interventions, and current pregnancy or breast feeding as well as obese and overweight patients with a body mass index > 25 were excluded. All patients were fully informed of the stages, possible complications, and postoperative care. The patients were randomized into 2 groups of 10 patients each: those who underwent transaxillary augmentation mammaplasty without and with endoscopic assistance, respectively. Round, high-profile silicone implants between 205 and 305 mL were implanted into all patients in the subglandular plane.
Surgical Procedure
The following were marked while the patient was sitting or standing: the submammary sulcus, anterior axillary line, midline in the anterior thoracic region, and incision, which must be positioned in the anterior axillary fold (Figure 1 A). The procedure was performed under locoregional anesthesia while the patient was the dorsal decubitus position with arms abducted. The patient's back was raised approximately 15º such that the sternal manubrium was horizontal, facilitating dissection and manipulation of the instrument12. A first-generation cephalosporin was administered as a prophylactic antibiotic. Both breasts were infiltrated with a 40 mL 0.5% lidocaine, 160 mL 0.9% physiological saline solution, and adrenaline (1:200,000).
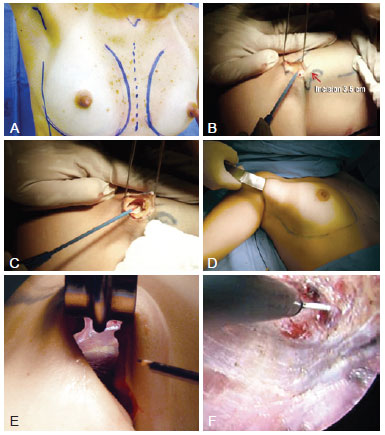
Figure 1 - Operative procedure. In A, marking of the anatomical parameters including the midline, submammary sulcus, and anterior axillary line. The scar must be positioned in the anterior axillary fold. In B, 3-4-cm incision in the anterior axillary fold. In C, subcutaneous dissection, which must be performed until the lateral edge of the pectoralis major can be identified. Deep dissection of the axillary fat must be avoided in order to prevent lesions in the neurovascular and lymphatic structures. In D, blunt dissection of the mammary gland. In E, view of the distal part of the mammary gland on the submammary sulcus without endoscopic assistance. It is important to use long materials for safe dissection. In F, dissection of the submammary sulcus with an endoscopically assisted frontal view and magnified structures.
A 3.5-4-cm incision was made in the anterior axillary fold (Figure 1 B). In patients who did not have well-defined axillary folds, the scar was positioned approximately 1 cm from the lateral edge of the pectoralis major muscle in the highest possible position in the axillary furrow such that it was invisible when the arms were abducted. Subcutaneous dissection was performed until the lateral edge of the pectoralis major muscle was visible. Here, caution must be taken to ensure the dissection is as superficial as possible in order to keep the axillary lymphatic structures intact (Figure 1 C). After identifying the lateral edge of the pectoralis major muscle, the mammary gland was detached from the suprafascial plane. The subcutaneous tunnel must provide sufficient space for the implant to be inserted but must not be dissected much in order to prevent cephalic migration of the prosthesis.
In the procedures performed without endoscopic assistance, the dissection was performed from the right view by using fiber optics, electrocautery, and blunt dissector (Figure 1 D). All instruments must be sufficiently long for appropriate dissection, primarily for the region below the papillary-areolar complex (i.e., the inferior internal and external quadrants) and more distal areas of the incision of greater technical difficulty (Figure 1 E). In the procedures performed with endoscopic assistance, the tower of the apparatus was positioned at the patient's foot so that the surgeon could move freely around the operating table. In addition to the materials already mentioned, a 30-gauge optical endoscopic retractor, aspirator, and dissectors (Karl Storz) were used. Up to the height of the PAC, the procedure was the same as that without endoscopic assistance. However, from this point onwards, the procedure was performed with endoscopic assistance (Figure 1 F).
After the implant was inserted, the mammary gland was closed with 3-0 nylon thread to prevent contact between the prosthesis and wound. The wound was closed with subcutaneous 3-0 nylon sutures, and subdermal and intradermal sutures were made with 4-0 Monocryl. Aspiration drains were not used. The dressing included gauze, sterile micropore tape, and bandages. The patients were discharged 24 hours postoperatively after the dressings were changed and a surgical bra was fitted along with a compressing band at the upper pole of the breast to prevent superior migration of the implants. The band and bra were kept on for 30 and 45 days, respectively.
The following parameters were assessed: operative time, complications, and return to normal daily activities. In the immediate postoperative period, the patients filled out a questionnaire about pain levels ranging from 1-5: grade 1, no pain; grade 2, discomfort without the need for medication; grade 3, pain requiring medication irregularly without restricting daily activities; grade 4, pain requiring regular medication, restricting but not preventing daily activities; and grade 5, pain preventing daily activities.
In the 6-month postoperative period, the patients answered questionnaires about the grade of satisfaction regarding the esthetic results of the breasts and scarring (Table 1) as well as loss of sensitivity in the breasts and PAC. Finally, surgeons who were uninvolved in the procedure assessed esthetic parameters including symmetry and positioning of the PAC and sulcus. All patients were assessed by the same surgeons. The non-parametric Mann-Whitney U-test was used for statistical analysis.
RESULTS
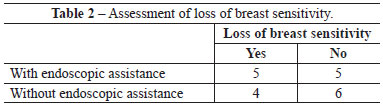
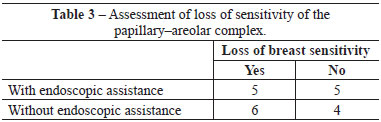
Among patients who underwent the procedure without endoscopic assistance, the mean operative time was 1 hour and 15 minutes. There were no cases of hematoma, seroma, infection, or capsular contracture. One case of dehiscence (0.5 cm) was observed in the postoperative wound, with spontaneous closure and without major repercussions on scar quality. Regarding postoperative pain, 7, 2, and 1 patient reported grade 3, 4, and 5 pain, respectively. All patients experienced an improvement in pain after approximately 1 week. Regarding loss of breast sensitivity, 6 patients experienced partial loss of sensitivity, 5 of whom recovered within 6 months and 1 who did not. Regarding sensitivity of the PAC, 4 patients experienced loss of sensitivity, which recovered within 6 months in all cases. Regarding the degree of satisfaction of breast esthetics, 7 and 3 patients were very satisfied (grade 5) and satisfied (grade 4), respectively. Regarding the esthetic result of the scar, 6 patients were very satisfied (grade 5), 3 patients were satisfied (grade 4), and 2 were satisfied but would not undergo the same incision again (grade 3).
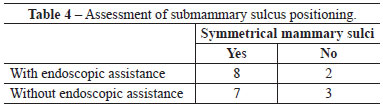
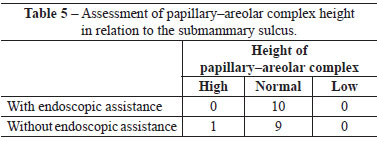
When assessed by other surgeons, the sulci were deemed well positioned and symmetrical in 7 out of 10 operated patients, whereas 3 patients were considered asymmetrical with respect to the positioning of the submammary sulcus. Regarding PAC height, no case was assessed as low or high (Figure 2).
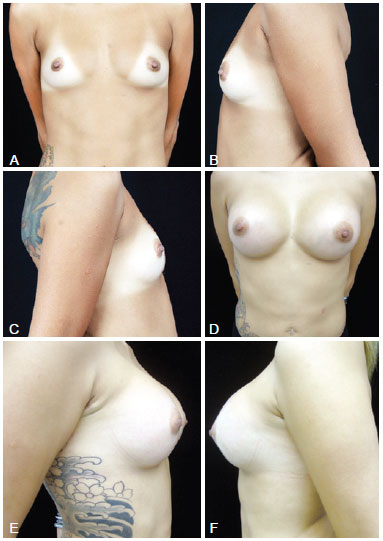
Figure 2 - In A, preoperative frontal view of a 26-year-old candidate for augmentation mammaplasty. In B, preoperative left lateral view. In C, preoperative right lateral view. In D, frontal view 6 months after transaxillary augmentation mammaplasty without endoscopic assistance with high-profile 280-mL silicone implants. In E, right lateral view 6 months postoperatively. In F, left lateral view 6 months postoperatively.
Among patients who underwent the procedure with endoscopic assistance, the mean operative time was 2 hours and 5 minutes. There were no cases of hematoma seroma, infection, or capsular contracture. There was one case of surgical wound hematoma that did not contact the mammary gland; the hematoma was drained in outpatient settings, without major repercussions on scar quality. Regarding postoperative pain, 1, 3, 5, and 1 patients experienced grade 1, 3, 4, and 5 pain, respectively. All patients experienced an improvement in pain after approximately 1 week. Regarding loss of breast sensitivity, 5 patients experienced partial loss of sensitivity, which recovered within 6 months in all cases. Regarding sensitivity of the PAC, 5 patients experienced loss of sensitivity, which returned within 6 months in all cases. Regarding the degree of satisfaction of breast esthetics, 3 patients were very satisfied (grade 5), 4 patients were satisfied (grade 4), and only 2 patients were satisfied but would not undergo the procedure again (grade 3). Regarding the esthetic result of the scar, 3, 5, and 2 patients were very satisfied (grade 5), satisfied (grade 4), and satisfied but would not undergo the same procedure again (grade 3).
When assessed by other surgeons, the sulci were deemed well positioned and symmetrical in 8 out of 10 operated patients, whereas 2 patients were considered to have asymmetric positioning of the submammary sulcus. Regarding the PAC height, 9 patients were considered to have a normal PAC height regarding the submammary sulcus, while only 1 patient exhibited a low height of the PAC in relation to the sulcus (Figure 3).
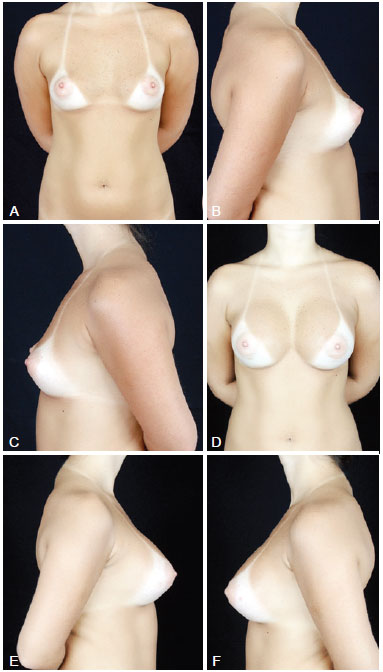
Figure 3 - In A, preoperative frontal view of a 32-year-old candidate for augmentation mammaplasty. In B, preoperative right lateral view. In C, preoperative left lateral view. In D, frontal view 6 months after transaxillary augmentation mammaplasty operation with endoscopic assistance with high-profile 305-mL silicone implants. In E, postoperative right lateral view. In F, postoperative left lateral view.
There were no significant differences between groups with respect to any parameter analyzed (Figures 4-6 and Tables 2-5).
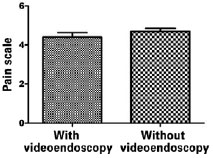
Figure 4 - Analysis of pain levels by non-parametric Mann-Whitney U-test.
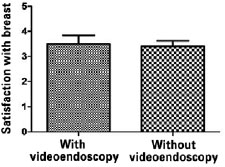
Figure 5 - Analysis of satisfaction regarding the esthetic results of the breasts by non-parametric Mann-Whitney U-test.
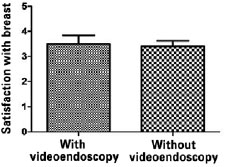
Figure 6 - Analysis of satisfaction regarding the esthetic results of the scar by non-parametric Mann-Whitney U-test.
DISCUSSION
Since the transaxillary procedure was first described, it has been recognized to have advantages including a lack of visible scarring on the breasts, which prevents surgical stigma in this region, and leaving the glands intact, which prevents possible lesions to the lymphatic ducts. Numerous studies demonstrate sufficient knowledge about the axillary structures and superficial dissection in this topography essentially guarantee a successful procedure without lesions in lymphatic drainage structures13,14. However, the performance of this procedure without endoscopic assistance requires some clarification, particularly regarding the greater rate of complications due to blind detachment, including edema and hematoma, and poorer esthetic results associated with less accuracy of dissection in the lower breast pole. The endoscopically assisted procedure was introduced as a safer alternative; it provides greater control of dissection and bleeding, producing more predictable results, fewer complications, and better esthetic results15,16. The benefits of the endoscopically assisted procedure include much smaller scars, less postoperative pain, and a shorter postoperative recovery period17.
Studies comparing these techniques have assessed the advantages and disadvantages of the different types of incisions and insertion planes of the implants, including operative time, patient satisfaction, esthetic results, and complication rates. Thus, the present study assessed these parameters in the same surgical procedure with or without endoscopic assistance.
The age range of the patients covered fertile women in their reproductive years. Thus, the sample is consistent with the average age of adults who undergo this procedure. As the sample size of each group was relatively small, group homogeneity was guaranteed and bias was minimized by applying rigorous inclusion and exclusion criteria.
The degree of patient satisfaction was assessed on a scale from 1-5 according to the parameters established by the authors. Despite being previously validated, the client satisfaction questionnaire (CSQ-8)18 was not used in this study, because the patients had difficulty answering it possibly owing to the sheer number of questions. Therefore, the author opted for more direct and short questions to assess the breasts and scars. Pain level including the use of medication and the performance of daily activities was also assessed on a scale from 1-5, which was well understood and answered by the patients.
The patients who underwent the procedure with or without endoscopic assistance also answered questions regarding return to activities of daily living. To avoid any bias, the same surgeons, who were uninvolved in the procedure, performed an esthetic analysis; the individuals' responses were kept completely confidential. In addition, basic postoperative assessment parameters were used, such as symmetry of the submammary sulci and PAC height. There were no major postoperative complications in any patient, including hematoma, seroma, or capsular contracture; there was only 1 minor complication in each group.
All of the criteria mentioned above were compared between groups by using the non-parametric Mann-Whitney U-test, because the distribution of data was non-normal. Accordingly, there were no significant differences between groups, including pain, complications, return to daily activities, esthetics, patient satisfaction, or the assessment criteria of anatomical parameters.
In the present study, the use of endoscopy extended operative time by at least 1 hour. Although this did not result in a higher complication rate, or affect esthetics or the postoperative stage of patients, it increased demands for surgical materials as well as trained personnel capable of performing the procedure, as mentioned by Munhoz et al.9.
The technique was performed according to guidelines with comprehensive anatomical knowledge of the axillary region and breast. The scar in the axilla was positioned appropriately, followed by the superficial dissection of the axillary fat, preventing lesions in the intercostobrachial3 branches. Moreover, sharp dissection below the PAC minimized bleeding at distal areas, and the blunt detachment was made 1 cm below the submammary sulcus, guaranteeing good postoperative results regardless of whether the procedure was executed with or without endoscopic assistance. In addition, the endoscope-assisted technique requires a slightly longer learning curve and increases the cost of augmentation mammaplasty.
CONCLUSIONS
There were no differences in postoperative parameters between transaxillary augmentation mammoplasties performed with or without endoscopic assistance. Endoscopic transaxillary augmentation mammaplasty without endoscopic assistance does not make the procedure safer or more predictable, but requires a greater learning curve and a higher operational cost compared to the procedure without endoscopic assistance.
REFERENCES
1. Hoehler H. Breast augmentation: the axillary approach. Br J Plast Surg.1973;26(4):373-6.
2. Graf RM, Bernardes A, Auersvald A, Damasio RC. Subfascial endoscopic transaxillary augmentation mammaplasty. Aesthetic Plast Surg. 2000;24(3):216-20.
3. Tebbetts JB. Axillary endoscopic breast augmentation: processes derived from a 28-year experience to optimize outcomes. Plast Reconstr Surg. 2006;118(7 Suppl):53S-80S.
4. Höhler H. Further progress in the axillary approach in augmentation mammaplasty: Prevention of incapsulation. Aesthetic Plast Surg.1977;1(1):107-13.
5. Tebbetts JB. Transaxillary subpectoral augmentation mammaplasty: a 9-year experience. Clin Plast Surg.1988;15(4):557-68.
6. Momeni A, Padron NT, Föhn M, Bannasch H, Borges J, Ryu SM, et al. Safety, complications, and satisfaction of patients undergoing submuscular breast augmentation via the inframammary and endoscopic transaxillary approach. Aesthetic Plast Surg. 2005;29(6):558-64.
7. Giordano PA, Rouif M, Laurent B, Mateu J. Endoscopic transaxillary breast augmentation: clinical evaluation of a series of 306 patients over a 9-year period. Aesthet Surg J. 2007;27(1):47-54.
8. Kolker AR, Austen WG Jr, Slavin SA. Endoscopic-assisted transaxillary breast augmentation: minimizing complications and maximizing results with improvements in patient selection and technique. Ann Plast Surg. 2010;64(5):667-73.
9. Munhoz AM, Fells K, Arruda E, Montag E, Okada A, Aldrighi C, et al. Subfascial transaxillary breast augmentation without endoscopic assistance: technical aspects and outcome. Aesthetic Plast Surg. 2006;30(5):503-12.
10. Niechajev I. Improvements in transaxillary breast augmentation. Aesthetic Plast Surg. 2010;34(3):322-9.
11. Regnault P. Breast ptosis. Definition and treatment. Clin Plast Surg. 1976;3(2):193-203.
12. Giordano PA, Rouif M, Laurent B, Mateu J. Endoscopic transaxillary breast augmentation: clinical evaluation of a series of 306 patients over a 9-year period. Aesthet Surg J. 2007;27(1):47-54.
13. Munhoz AM, Aldrighi C, Ono C, Buchpiguel C, Montag E, Fells K, et al. The influence of subfascial transaxillary breast augmentation in axillary lymphatic drainage patterns and sentinel lymph node detection. Ann Plast Surg. 2007;58(2):141-9.
14. Weck Roxo AC, Aboudib JH, De Castro CC, De Abreu ML, Camões Orlando MM. Evaluation of the effects of transaxillary breast augmentation on sentinel lymph node integrity. Aesthet Surg J. 2011;31(4):392-400.
15. Momeni A, Padron NT, Föhn M, Bannasch H, Borges J, Ryu SM, et al. Safety, complications, and satisfaction of patients undergoing submuscular breast augmentation via the inframammary and endoscopic transaxillary approach. Aesthetic Plast Surg. 2005;29(6):558-64.
16. Momeni A, Padron NT, Bannasch H, Borges J, Björn Stark GB. Endoscopic transaxillary subpectoral augmentation mammaplasty: a safe and predictable procedure. J Plast Reconstr Aesthet Surg. 2006;59(10):1076-81.
17. Pacella SJ, Codner MA. The transaxillary approach to breast augmentation. Clin Plast Surg. 2009;36(1):49-61.
18. Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann. 1982;5(3):233-7.
Plastic surgeon, full member of the Brazilian Society of Plastic Surgery, Rio de Janeiro, RJ, Brazil
Correspondence to:
Ana Claudia Weck Roxo
Rua Ipanema, 21/1.803 - Barra
Rio de Janeiro, RJ, Brazil - CEP 22631-390
E-mail: anacwroxo@rjnet.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: 13/7/2012
Article accepted: 21/9/2012
Study undertaken at the author's private clinic, Rio de Janeiro, RJ, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter