

Original Article - Year 2013 - Volume 28 -
Brazilian version of the Breast Evaluation Questionnaire: cultural adaptation and validation
Tradução para a língua portuguesa, adaptação cultural e validação do Breast Evaluation Questionnaire
ABSTRACT
BACKGROUND: Measurable data on plastic surgery outcomes are scarce. In recent years, questionnaires to measure quality of life have been used globally. In Brazil, there are no questionnaires validated and adapted in the Brazilian population that specifically assess quality of life after breast surgery. The aim of this study was to translate the Breast Evaluation Questionnaire (BEQ 55) into Portuguese, and culturally adapt and validate the translation for use in Brazil.
METHODS: Two translations, two revisions by a multidisciplinary group, and two back translations of the questionnaire were performed. Cultural adaptation was performed by applying the questionnaire to groups of 20 patients from the plastic surgery outpatient clinic. The questionnaire included relevant modifications for better understanding of the questions. To test the questionnaire's reproducibility and validity, 20 patients were interviewed on two separate occasions. On the first occasion, they were interviewed by different interviewers, and on the second occasion (after 7 days and after 14 days), by only one. In addition, the Short-Form 36 was applied during the first interview.
RESULTS: During cultural adaptation, questions were modified to facilitate the patients' understanding. A new group was tested to confirm that items were understood. Internal consistency of the questionnaire ranged between 0.931 and 0.936. The interobserver reproducibility coefficient was 0.962, and the intraobserver reproducibility coefficient was 0.919. Only the domains of the SF-36 regarding functional capacity, general health status, and emotional aspects correlated with the total score of the BEQ 55.
CONCLUSIONS: The BEQ 55 questionnaire was successfully translated and adapted. The Brazilian version was called "Questionário de Avaliação das Mamas (BEQ-Brasil)" and was demonstrated to be valid and reproducible.
Keywords: Breast/surgery. Quality of life. Plastic surgery. Questionnaires.
RESUMO
INTRODUÇÃO: Dados mensuráveis de resultados em cirurgia plástica são escassos. Nos últimos anos, instrumentos de medida de qualidade de vida vêm sendo utilizados em escala mundial. Não há instrumentos válidos e adaptados no Brasil para avaliar qualidade de vida especificamente para cirurgia das mamas. O objetivo deste estudo é traduzir para o português, adaptar culturalmente e validar o Breast Evaluation Questionnaire (BEQ 55) para uso no País.
MÉTODO: Foram realizadas duas traduções e duas traduções reversas do instrumento, intercaladas por revisões de comitê multidisciplinar. A adaptação cultural foi feita com aplicação do questionário a grupos de 20 pacientes do ambulatório de cirurgia plástica, com modificações pertinentes para melhora do entendimento. Para testar a reprodutibilidade e a validade de construção, 20 pacientes foram entrevistados em duas ocasiões: na primeira, por entrevistadores diferentes, e na segunda (após 7 dias a 14 dias), por apenas um deles. Na primeira, foi aplicado também o Short-Form 36.
RESULTADOS: Na adaptação cultural, foram modificadas todas as questões para facilitar o entendimento. Um novo grupo obteve boa compreensão de todas as questões. A consistência interna do instrumento variou de 0,931 a 0,936. O coeficiente de reprodutibilidade interobservador foi de 0,962 e o intraobservador, de 0,919. Apenas os domínios do SF-36 capacidade funcional, estado geral de saúde e aspectos emocionais tiveram correlação com o escore total do BEQ 55.
CONCLUSÕES: O questionário foi traduzido e adaptado com sucesso, sendo a versão brasileira denominada Questionário de Avaliação das Mamas (BEQ-Brasil), e provou ser válido e reprodutível.
Palavras-chave: Mama/cirurgia. Qualidade de vida. Cirurgia plástica. Questionários.
In the past, the assessment of outcomes in plastic surgery relied on subjective measures, such as the comparison of photographic images1. Aesthetic and reparative surgery procedures now achieve significant improvements in quality of life2, and thus questionnaires to assess this can be used for the objective assessment of outcomes. The questionnaires used to perform this assessment can be generic, such as the Short-Form Health Survey (SF 36), or specific to a specific condition, such as the Breast Evaluation Questionnaire (BEQ). A systematic review of the different types of questionnaires to assess aesthetic surgery outcomes - in terms of validity, reliability, and sensitivity - showed that those that measure quality of life and self-image are the most appropriate1.
The BEQ includes 55 questions on a women's satisfaction and comfort with breast appearance3. However, to date it was presented and validated only in the English language. For use in Brazil, it was therefore necessary to translate it into Portuguese and to adapt it culturally and validate it for use. This translation process has been scientifically standardized through similar adaptations of questionnaires for psychology and sociology studies4.
The aim of this study was to translate the BEQ 55 into Portuguese, adapt it for use in the Brazilian context, and validate it for use in Brazilian women.
METHODS
This study included 60 female patients aged between 18 and 60 years who attended the Outpatient Breast Clinic of Plastic Surgery at the Escola Paulista de Medicina, Federal University de São Paulo. This study was approved by the Research Ethics Committee of the Universidade Federal de São Paulo under protocol 1817/07.
Translation
Translation from English into Portuguese was performed by two independent translators and both versions were assessed by a multidisciplinary team of two plastic surgeons and a rheumatologist in order to reach a consensus version. Then this version was back translated by two other translators. The last translation was compared to the original version of the questionnaire by the same multidisciplinary group and a second Portuguese version was produced.
Cultural Adaptation
The second Portuguese version was tested on 20 patients in the study group (pre-test 1 group) to detect translation errors in the questionnaire and to assess patient comprehension. Any items that were not understood by more than 20% of patients were revised by the multidisciplinary group, and a third version was prepared and tested on a new group of 20 patients (pre-test 2 group). This version was understood by more than 20% of the interviewees and was kept as the final version.
Reproducibility
The final version of the questionnaire was tested on another group of 20 patients (reproducibility group) by two independent interviewers on two separate occasions. On the first occasion, they were interviewed by different interviewers, and on the second occasion (after 7 days and after 14 days), by only one.
Internal Consistency
The internal consistency of the questionnaire was tested using the Cronbach's alpha coefficient. The homogeneity of the items was analyzed using the correlation between each item and the total score of the corresponding scale.
Validity
Internal validity was tested using the SF 36 and the BEQ 55 questionnaires during the first interview with the reproducibility group.
Analysis of the Results
Intraclass correlation coefficients (ICCs) were calculated to assess the reproducibility of the questionnaire. The ICC (1,1) was used to calculate intraobserver reproducibility, and the ICC (2,1) was used to calculate interobserver reproducibility5.
The Pearson's coefficient of linear correlation was calculated to test the relationship between continuous variables.
The scores of the SF 36 scale were compared with the sub-items of the BEQ scale using cluster analysis to identify groups with similar characteristics. Statistical significance was set at P ˂ 0.05. All tests were two-tailed. A 95% confidence interval (CI) was calculated for the ICCs. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) 13.0 software for Windows.
RESULTS
Cultural Adaptation
Questions 3 and 4 of the BEQ 55 were modified to make them more culturally appropriate, and the indications included at the end of the questions were expressed as sub-items to facilitate patient understanding (Figure 1). These changes resulted in a third translated version of the questionnaire, which was the final version (Figure 2).

Figure 1 - Example of a question that was modified to facilitate the patients' understanding, which was included in the final version of the questionnaire.

Figure 2 - Final version of the questionnaire.
Reproducibility
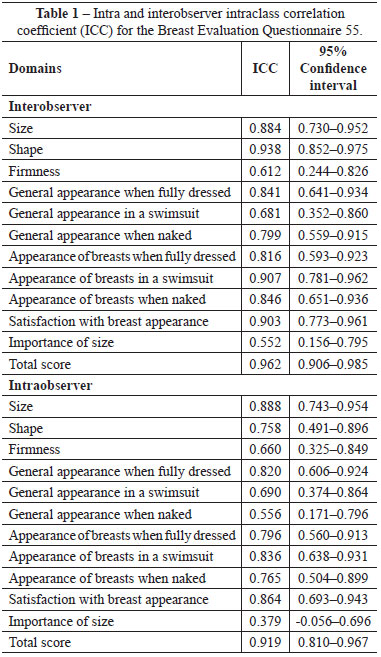
The intraobserver ICC was 0.919, indicating agreement between the scores of the interviews conducted by the same interviewer on separate occasions, and the interobserver ICC was 0.962, indicating agreement between the scores of the interviews conducted by different interviewers on the same occasion (Table 1).
Internal Consistency
The Cronbach's alpha coefficients confirmed that the questionnaire had internal consistency, as they were 0.936 for the first interview, 0.931 for the second interview, and 0.935 for the third interview.
Validity
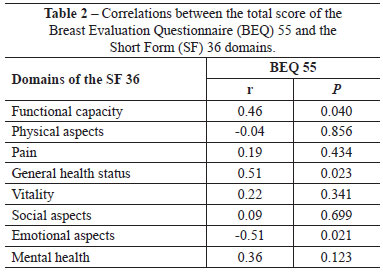
Pearson's coefficients of linear correlation were calculated to investigate the correlation between the total score of the BEQ and the items of the SF 36 (Table 2).
DISCUSSION
Studies on patient satisfaction with augmentation mammaplasty have been conducted for more than three decades6. However, the first studies were mostly retrospective, un-controlled, and based on simple questions or using questionnaires that were not validated. Quality of life has been assessed more often since the 1990s, and most studies that assessed this parameter after augmentation mammaplasty used the SF 361,7.
The BEQ 55 is a self-administered questionnaire with 55 questions. It evaluates patient satisfaction with their breasts and changes in quality of life after breast surgery. Responses are rated on a 5-point scale, with 1 equal to "very dissatisfied or very uncomfortable" and 5 equal to "very satisfied or very comfortable" and consists of three parts. The first part includes questions on satisfaction with size, shape, and firmness of the breasts in distinct situations: intimate, social, or professional activities. The second part relates to the degree of comfort with general appearance or specific appearance of the breasts in several contexts: when fully dressed, in a bathing suit, or naked, and while alone, with a sexual partner, with men in general, with women they know, with women in general, or with health professionals. The third part has two questions; the first asks about the level of satisfaction with the appearance of the breasts for the patients themselves, the spouse or partner, parents, siblings, and friends. The last question determines the importance of breast size for the patient themselves and for the people they know3.
Anderson et al.3 tested the validity of the BEQ 55 by comparing it with other questionnaires, and found that the three parts of the BEQ 55 correlated better with each other than with any other scale. Therefore, it measured something different from, but related to the other measures3. In this study, we obtained comparable results when comparing BEQ 55 with SF-36.
The Guillemin et al.4 method is the most accepted internationally for the translation of instruments of measure. The main stages include the following: doing more than one translation, revision by a multidisciplinary group to test semantic equivalence, comparison with back translations into the original language, and cultural adaptation to the target population. We believe that this method makes the questionnaire more reliable.
During cultural adaptation of the BEQ 55 to the Brazilian population, we found that the patients' low level of education was the reason why simple expressions such as "swimwear" were not understood and had to be modified. Moreover, indirect sentences were only understood by a few patients and were replaced by direct sentences. For example, the third question "How satisfied with the general appearance of your breasts are the following people in your life?" was replaced by "Are you and the people in your life satisfied with the (visual) appearance of your breasts?"
The SF-36 questionnaire was used for comparison because it is the most used questionnaire in the literature to assess changes in the quality of life after breast surgery, and because there is no specific validated clinical questionnaire for breast assessment in Brazil. The questionnaires used for the validation of the American version do not have a Portuguese version. The domains relating to functional capacity, general health status, and emotional status were most strongly correlated with the total BEQ 55 score. However, the emotional aspects correlated negatively, i.e., those patients with more emotional problems were apparently less concerned with their breasts.
Scores were standardized as percentages, as the score range for each sub-item is different. The minimum score value of each sub-item was subtracted from the net score. This result was divided by the possible variation of the score and multiplied by 100 to get a percentage.
The best BEQ 55 scores were obtained for satisfaction with breast attributes, and general and breast appearance when fully dressed. The worst BEQ 55 scores were obtained for general appearance in a bathing suit and when naked, as well as breast appearance when naked. These data reflected the characteristics of the study population, i.e., most patients were older than 40 years of age, overweight, and recovering from breast reconstruction.
Both intraobserver and interobserver reproducibility (ICC) for the total scores of the questionnaire exceeded 0.9; reproducibility should exceed 0.5 for a questionnaire to be deemed reliable1. The internal consistency of the questionnaire was high, and a value of ˃ 0.71 is considered satisfactory.
The author of the original questionnaire suggested that this questionnaire should also be applied to patients who undergo surgery for breast cancer, reduction mammaplasty, and breast reconstruction. Moreover, the author highlighted the importance of developing questionnaires that are specific for not only breast reconstruction but also for each type of breast surgery, because although they have a common basis, the desired outcomes for the patient are not necessarily the same8.
The questionnaire does not address important topics suggested by other authors that are relevant to patients undergoing other types of breast surgery, such as physical symptoms of breast hypertrophy, scars, appearance of the nipple-areola area, sensitivity, symmetry, libido, and physical exercise9. However, some of these factors may influence a patient's responses to the BEQ 55.
It is possible that responses to the BEQ 55 regarding surgery-specific questionnaires will be lower; however, it may be very useful to compare different groups. Therefore, more studies are needed to validate the BEQ 55 questionnaire across different populations of patients and to observe the responses in patients who have undergone different types of surgery. The present study allows other studies to be performed in Brazil because it provides a Portuguese version of the BEQ 55 questionnaire that was prepared according to the internationally accepted methodology.
CONCLUSIONS
The Breast Evaluation Questionnaire was successfully translated into Portuguese and adapted for use in Brazilian women. The Brazilian version is called "Questionário de Avaliação das Mamas (BEQ 55 - Brasil)" and has been demonstrated to be a valid and reproducible questionnaire.
REFERENCES
1. Ching S, Thoma A, McCabe RE, Anthony MM. Measuring outcomes in aesthetic surgery: a comprehensive review of the literature. Plast Reconstr Surg. 2003;111(1):469-80.
2. Klassen A, Jenkinson C, Fitzpatrick R Goodacre T. Patients' health related quality of life before and after aesthetic surgery. Br J Plast Surg. 1996;49(7):433-8.
3. Anderson RC, Cunningham B, Tafesse E, Lenderking WR. Validation of the breast evaluation questionnaire for use with breast surgery patients. Plast Reconstr Surg. 2006;118(3):597-602.
4. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46(12):1417-32.
5. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-8.
6. Hetter GP. Satisfactions and dissatisfactions of patients with augmentation mammaplasty. Plast Reconstr Surg. 1979;64(2):151-5.
7. Veiga DF, Sabino Neto M, Ferreira LM. Quality of life outcomes after pedicled TRAM flap delayed breast reconstruction. Br J Plast Surg. 2004;57(3):252-7.
8. Pusic AL, Klassen A, Cano SJ, Kerrigan CL. Validation of the breast evaluation questionnaire. Plast Reconstr Surg. 2007;120(1):352-3.
9. Sabino Neto M, Freire M, Garcia EB, Ferreira LM. Functional capacity and postural pain outcomes after reduction mammaplasty. Plast Reconstr Surg. 2006;118(4 Suppl):117-21.
1. Plastic surgeon, associate member of the Sociedade Brasileira de Cirurgia Plástica (SBCP), Master in Plastic Surgery by the Escola Paulista de Medicina - Universidade Federal de São Paulo (Federal University of São Paulo - EPM-UNIFESP), Attending Physician at the Hospital Pérola Byington, São Paulo, SP, Brazil
2. Plastic surgeon, full member of the SBCP, Associate Professor of Plastic Surgery at the EPM-UNIFESP, Coordinator of the Postgraduate Program in Translational Surgery of the EPM-UNIFESP, São Paulo, SP, Brazil
3. Resident physician in Plastic Surgery at the EPM-UNIFESP, aspiring member in training of the SBCP, São Paulo, SP, Brazil
4. Plastic surgeon, full member of the SBCP, Master in Plastic Surgery at the EPM-UNIFESP, São Paulo, SP, Brazil
5. Plastic surgeon, full member of the SBCP, full professor of Plastic Surgery at the EPM-UNIFESP, São Paulo, SP, Brazil
Correspondence to:
Lia Fleissig Ferreira
Rua Pedro de Toledo, 541 - ap. 31 - Vila Clementino
São Paulo, SP, Brazil - CEP 04039-031
E-mail: liafleissig@gmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: February 2, 2013
Article accepted: June 29, 2013
This study was performed at the Escola Paulista de Medicina - Universidade Federal de São Paulo (Federal University of São Paulo), São Paulo, SP, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter