

Special Article - Year 2013 - Volume 28 -
Follicular unit megasessions and platelet growth factors
Megassessoes de unidades foliculares e fatores de crescimento plaquetario
ABSTRACT
Hair loss, whether partial or complete, is a cause of significant concern to both men and women, and is viewed as unaesthetic and a visible sign of aging. Advances and refinements in hair restoration techniques have culminated in the introduction of micrograft and minigraft megasessions. This technique has become widely accepted as a simple and safe procedure that recreates natural random-pattern hairlines. The hair follicles are harvested from the posterior cervical area, where 500 to 1500 follicular units can be obtained. Implantation in the bald area is performed via punctiform incisions using the No. 11 blade. After the procedure, gauze moistened in saline solution is applied over the implanted area for 24 hours. The stitches are removed on the 7th postoperative day. The final result is visible after 8 to 12 months in men and after 12 to 14 months in women. If an additional procedure is necessary, this can be performed 1 year after the initial transplantation. The quality and strength of the implanted hair persists for an indefinite period in some patients because of 1 the following particular characteristics: high histological quality of the donor area, heredity, hormones, or aging. This article also addresses the role of platelet-rich plasma growth factors in surgical treatment of male and female pattern baldness. The results of hair transplant surgery suggest that the use of autologous platelet growth factors improves capillary density. This offers a new perspective on hair transplantation and is an important contribution to implantation surgery with follicular unit megasessions.
Keywords: Hair follicle/transplantation. Scalp/surgery. Alopecia/surgery.
RESUMO
Queda de cabelo, sej a parcial ou completa, é causa de preocupação significativa para homens e mulheres, que a veem como um sinal inestético e visível de envelhecimento. Avanços e refinamentos das técnicas culminaram na introdução de megassessões de microenxertos e minienxertos. Essa técnica se tornou amplamente aceita como um procedimento simples e seguro, que recria as linhas randômicas naturais do cabelo. Os folículos pilosos foram retirados da área cervical posterior, onde 500 a 1.500 unidades foliculares podem ser obtidas. A área calva foi implantada através de incisões de lâmina nº 11. Após o procedimento, gaze umedecida em solução salina foi aplicada sobre a área implantada por 24 horas. Os pontos foram removidos no 7º dia de pós-operatório. O resultado final foi obtido 8 meses a 12 meses após o procedimento, em homens, e 12 meses a 14 meses, em mulheres. Nos pacientes em que um segundo procedimento foi necessário, este foi realizado 1 ano após o transplante inicial. A qualidade e a força do cabelo transplantado permanecem em alguns pacientes por tempo indeterminado, em decorrência de características particulares, como alta qualidade histológica da área doadora, hereditariedade, hormônios e envelhecimento. A cirurgia de transplante capilar demonstra que o uso de fatores de crescimento plaquetário autólogo pode melhorar a densidade capilar. Esse processo oferece uma nova perspectiva ao transplante capilar, representando uma contribuição importante para a cirurgia de implante com megassessões de unidades foliculares.
Palavras-chave: Folículo piloso/transplante. Couro cabeludo/cirurgia. Alopecia/cirurgia.
Hair loss, whether partial or complete, is a cause of significant concern to both men and women, and is viewed as unaesthetic and as a visible sign of aging. The significant increase in the demand for hair restoration has resulted in improvements in hair transplantation techniques.
EVOLUTION OF TECHNIQUE
Reports of modern hair transplantation can be traced back as early as 1940. In 1959, Orentreich1 introduced the use of punch grafts and the concept of donor dominance, in which individual hair follicles preserve their own hereditary characteristics after transplantation, thus enabling hair growth at the recipient site. Temporoparietooccipital (TPO) flaps, which are used to improve the aesthetic appearance of transplanted hair, were introduced by Juri2 and were subsequently improved by Chajchir3 et al and Uebel4. Nordström5 and Marrit6 introduced the micrograft concept to camouflage the disadvantages of burns and residual scars. Further advances and refinements culminated in the introduction of micrograft and minigraft megasessions in 1986 by Uebel4,7-14. This technique has become widely accepted as a simple and safe procedure that recreates natural random-pattern hairlines with feathered natural borders.
INDICATIONS AND CONTRAINDICATIONS
Male baldness, which is associated with aging and becomes more evident after the age of 30 years, is related to 3 main factors: heredity, androgenic hormones, and aging. Hair loss occurs during menopause in women, but androgenic female alopecia can occur at an earlier age. Careful patient selection and good communication are crucial. Patients must have realistic expectations and understand that the method will redistribute their available hair; success depends on the density and quality of the hair to be transplanted, and a second replacement procedure will be required to maintain hair density if the nature of their baldness is progressive. The male patient seeks to reverse hair loss, whereas in women, hair transplantation is indicated for androgenic baldness, reconstructing sideburns after a face-lift, and improving temporal recesses. Surgery is not recommended for individuals with diabetes or coagulation disorders in whom the prothrombin level is below 75%.
PREOPERATIVE ASSESSMENT AND EVALUATION OF CHARACTERISTICS
Obtaining a complete history and performing laboratory tests are standard evaluation procedures. Griffin15 established 4 characteristics of the hair:
color (black, brown, red, blond, or gray); texture (coarse, medium, or fine); skin-to-scalp contrast (high, medium, or low); degree of curl (kinky, curly, or straight).
Norwood16 added 2 factors (density and size of the donor area) and Uebel17 added 2 other hair types (white and wavy hair). Density is an important factor, and younger patients have denser hair. Coarse hair usually covers more effectively than fine hair, but shafts of thick hair tend to be more visible. Although fine shafts achieve a better hairline and the aesthetic results are superior to those with coarse hair, these shafts often require repeated procedures because the hair density is lower. Wavy and kinky hair types provide better coverage of the bald area. An oily surface with transplanted black hair causes high contrast, resulting in an artificial appearance; thus, a random distribution of implants is recommended. The contrast against the scalp is lower with brown and gray hairs compared to black hair, and the result is more natural. Red hair maintains its color or lightens with age, giving patients a youthful appearance. The best results are observed with blond hair because it is usually fine and the contrast to the scalp is minimal. In most cases, 2 or 3 procedures must be performed to achieve good density. Although it is very difficult to work with the roots of white hair because they are almost imperceptible during preparation, the aesthetic result is very pleasing. Kinky and curly hair types cover and protect the scalp better than other kinds of hair, resulting in superior density and aesthetic results. Wavy hair provides the patient with different hairstyle options and good natural density. The aesthetic result from straight hair is good, but 2 procedures are usually required in such cases.
PHOTOGRAPHIC DOCUMENTATION AND HAIRLINE DESIGN
Pictures are very important for evaluating the new hairline, baldness area, and postoperative results. Images are obtained while the patient is in a seated position with the head at a 45-degree angle. Pictures are obtained from the front, right, and left sides and from the crown area, and a close-up is obtained, if necessary. Postoperative pictures are obtained at 8 months and at 12 to 15 months after the procedure for men and women, respectively, at the same views and angles of the preoperative images. The anterior hairline should be irregular and asymmetrical18. The hairline is discussed with the patient in front of a mirror and the new irregular frontal hairline is drawn on the scalp, while maintaining temple recessions (Figure 1). Usually, the midpoint of the new hairline lies 8 cm from the glabella, in a VW irregular line up to the temporal recesses. The patient is given an explanation of the progressive nature of baldness and is informed that a second replacement procedure may be necessary. Honesty will increase the patient's confidence.
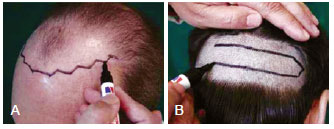
Figure 1 - In A, the new hairline is discussed with the patient in front of a mirror and is drawn on the forehead. An irregular frontal hairline will yield a more natural and aesthetic result. In B, the donor area is shaved and a hair-bearing ellipse is marked. The hair follicles are harvested from the posterior cervical area that has the best histological hair follicles and the best hair density. This area is normally 6 to 8 cm from the bottom.
ANATOMY
The growth cycle of hair has 3 phases: anagen (growing, adult phase), catagen (involution and loss), and telogen (preparation for new hair). The anatomic/histologic structure of the scalp consists of 2 to 4 terminal follicles, 1 or 2 vellus follicles, sebaceous glands, and insertions of the arrector pili muscles, which are surrounded by the perifolliculum (reticular dermis collagen fibers).
SURGICAL TECHNIQUE
The hair follicles are harvested from the posterior cervical area where the hair is dense and the histological capillary roots are of high quality. The hair-bearing ellipse flap varies from 10 × 2 cm to 20 × 3 cm in size (Figure 2). Here, 500 to 1500 follicular units can be obtained. Anesthesia includes sedation, supraorbital and occipital nerve blockage, and scalp ballooning. Scalp ballooning is a procedure described by Uebel9 that increases the edema of the scalp and causes ischemia, which facilitates hair implantation. All layers of the scalp and donor site are infiltrated with saline solution containing epinephrine (1:140.000). The hair-bearing ellipse is cut at an oblique angle to avoid damaging the follicles, and the flap is raised from the subcutaneous fat layer with the galea aponeurotica remaining intact. Nylon 4.0 subcutaneous and cutaneous sutures are placed while avoiding tension because the scalp does not tolerate stretching (Figure 2). The borders can be undermined, if necessary, while taking care not to damage the vessels and nerves. The follicular units are prepared on a hard wooden or acrylic surface (Figure 3). Excess subcutaneous fat is removed and is divided into micrografts (1 to 2 bulbs) and minigrafts (3 to 4 bulbs), both of which are called follicular units. The follicular units are separated with or without magnification by a skilled and experienced team. Larger interstices and the excess connective tissue surrounding the grafts are trimmed away. In the histological specimen, the follicles are observed as being separated by fibroconnective tissue forming units (Figure 4). The grafts are kept moist after they are prepared, and they should be transplanted within 20 minutes to prevent loss of quality. Tumescent infiltration of all layers of the scalp is subsequently performed to achieve sympathetic and microvascular plexus white marbling. The area is then ready for implantation through No. 11 sharp blade punctiform incisions, after which the assistant gently inserts the graft into the slit with angled microsurgical forceps. The blade is subsequently removed to facilitate insertion. This maneuver, called stick-and-place, is performed simultaneously (Figure 5). It is important to maintain a space of 4 to 5 mm between each unit to prevent the implanted units from projecting outward. If greater density is desired, the surgeon should wait for 15 to 20 minutes to ensure that the fibrin formed from fibrinogen has maintained the graft in place. Gauze moistened in saline solution is applied over the implanted area after the procedure, and a bandage is kept in place for 24 hours (Figure 6).
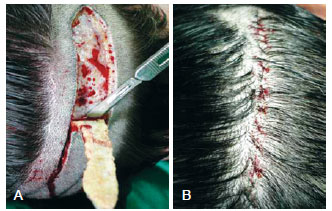
Figure 2 - In A, the hair-bearing ellipse is cut using a single No. 10 blade at an oblique angle to avoid damaging the hair follicles. In B, subcutaneous sutures are placed and a running superficial cutaneous suture is used to close the defect.
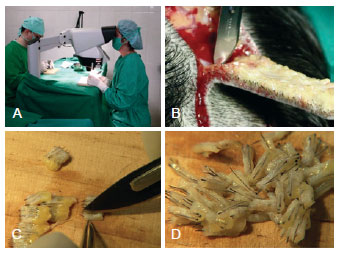
Figure 3 - In A, graft preparation using three-dimensional stereoscopic visualization. The use of magnifying lenses for the preparation of follicular units contributes to improve the quality of the graft. In B, the elliptical incision in the scalp at the donor area. In C and D, individualization and graft preparation.
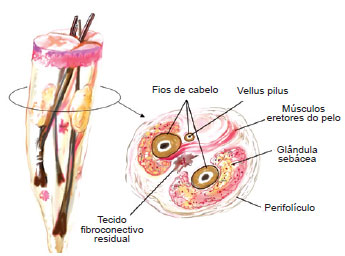
Figure 4 - The anatomic/histologic structure of the scalp was described by Headington. The scalp usually consists of 2 to 4 terminal follicles, 1 to 2 vellus follicles, sebaceous glands, and insertions of the arrector pili muscles.
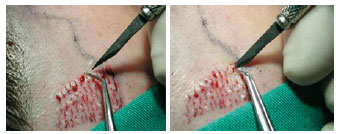
Figure 5 - A needle (40 X 12), a No. 11 sharp blade, or a microsurgical blade (Beaver 6500-BD) is used to make the punctiform incision. Angular microsurgical forceps is used to grasp the follicular units and bring them to the slit. The incision is 3 to 4 mm deep, and this depth is sufficient for gently placing the graft. Care must be taken not to cut the galea aponeurotica, as this would result in additional bleeding and the graft could be totally buried.
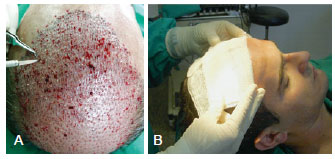
Figure 6 - The procedure for implantation of1,500 units follicle takes about 2 hours. In A, the aspect of the scalp after implantation of hundreds of follicular units. In B, gauze dressings moistened with saline solution is applied over the implanted area, and maintained for 24 hours.
Patients can be administered analgesics, but antibiotics are not routinely recommended. The patients are instructed to rest for 72 hours and to sleep with the head raised at a 30-degree angle to avoid swelling of the forehead, which may occur as a result of scalp ballooning. This swelling decreases in 48 hours. We recommend the use of an analgesic such as 10 mg of sublingual Toragesic® (Ketorolac), 3 times a day, and prednisone (40 mg on the day of surgery and 20 mg per day for the 2 following days). The patient may experience discomfort in the donor area for 6-8 hours after surgery.
The dressing is removed on the first postoperative day and the scalp is cleaned with a neutral shampoo. Bleeding may occur if any implant is dislodged during this procedure, and the bleeding can be stopped by pressing the area for 3 to 5 minutes.
The repair stitches are loosened and the running suture is removed on the 3rd and on the 7th postoperative days, respectively. It is very important to make the patient aware that the telogen phase begins after 1 to 3 weeks of discrete hair growth, and that almost all of the implanted hair will fall out during this phase. This phase continues for 6 to 9 weeks, after which the anagen phase begins and the matrix generates very thin and delicate new hair. The final result is visible only after 8 to 12 months in men and after 12 to 14 months in women; a second procedure can be performed after 15 months, if necessary.
It is common for cysts to appear after the third month; these should be ruptured using a sterilized needle or forceps and the scalp should be cleaned with antiseptic solution. If a second procedure is necessary, this can be performed 1 year after the initial transplantation. The surgical scar persists indefinitely and the quality is unpredictable.
Complications are rarely associated with this technique, and the complications that do occur are minor compared with those associated with other techniques. The most common complication involves forehead and eyelid edema, which develops 2 to 4 days postoperatively. A trial procedure is recommended for patients at a high risk for keloid scars. Donor and recipient sites are examined 6 to 12 months pos-toperatively to observe the healing process. Temporary alopecia may be observed around a tight donor site closure (the telogen effluvium phase induced by ischemia), with hair regrowth within 3 to 4 months.
The results of this procedure are shown in Figures 7 to 10.
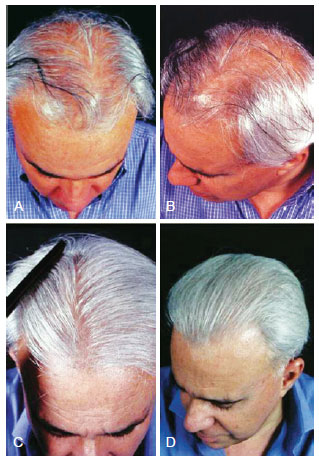
Figure 7 - A 48-year-old patient with hair loss and gray hair. In A and B, preoperative aspect. In C and D, 15 months postoperatively aspect, demonstrating good capillary density.
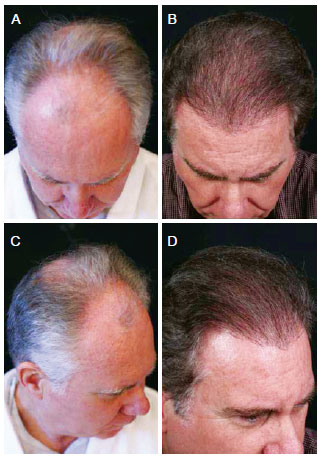
Figure 8 - A 62-year-old patient with hair loss and gray hair. In A and C, preoperative aspect. In B and D, 18 months postoperatively aspect, demonstrating good capillary density and colored hair.
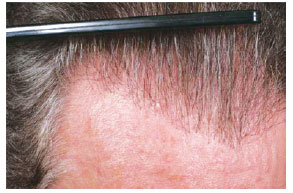
Figure 9 - Close up view of the same patient of the Figure 8.
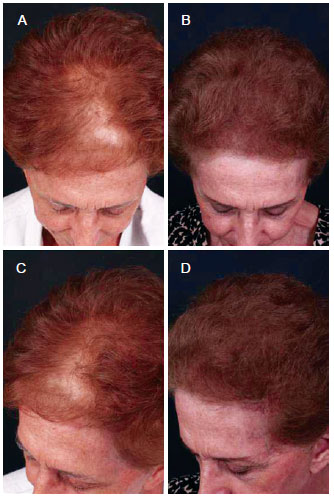
Figure 10 - A 73-year-old patient with hair loss underwent a single follicular units megasession implant. In A and C, preoperative aspect. In B and D, postoperative aspect.
DISCUSSION
Following its introduction, the megasessions method became a reference technique and it has been presented in many scientific works8,9,19. Many improvements have been made to this technique, and the introduction of new materials and laser technology has facilitated further advances. It is currently used widely by many surgeons worldwide and is the most common type of surgery for treating baldness.
Careful patient selection and good patient communication are crucial for achieving optimal results. A well-trained team is a requisite for a good outcome. Surgeons should always be honest when discussing the positive and negative aspects and should inform the patient that a second procedure may be necessary. It is essential that the diagnosis of this condition is performed systematically, thus leading to appropriate treatment. A series of studies regarding classification of this condition and improvements in surgical methodology in order to optimize the results have been published. These publications provide important new information about diagnosis algorithms, classification of alopecias, and useful systematizations, including patient positioning and types of incisions20-24.
The scalp ballooning tumescent technique is important for preventing bleeding during surgery and for maintaining the grafts within the punctiform incision. The surgeon should avoid damage of the sensitive branches in the donor region to prevent posterior paresthesias or pain, both of which can persist for months. It is recommended that a maximum of 1500 follicular units should be implanted per procedure.
The most common cause of female pattern baldness is androgenic alopecia, which usually occurs during menopause. The surgical technique is similar to that described for men, with a few modifications. Because women have finer hair compared to men, mini-follicular units containing 3 to 4 shafts are implanted rather than micro-units with 1 or 2 shafts.
THE ROLE OF PLATELET-RICH PLASMA GROWTH FACTORS IN SURGERY FOR MALE AND FEMALE PATTERN BALDNESS
Follicular units are commonly used in surgery to treat baldness, and they have been used globally to treat both male and female patients. The yield from micrografts, which varies between 70% and 85%, is determined by factors such as the quality of the harvested donor area, preparation of the units, care taken during the implantation procedure, and follicular apoptosis.
Autologous platelet-rich plasma (PRP) has been used in several experimental and clinical studies because of its benefits for stimulating cell proliferation and improving healing. PRP has also attracted attention in plastic surgery and dermatology because of its potential use during facial plastic surgery, procedures for aesthetic skin rejuvenation, and hair transplantation25-33.
Basically, 3 growth factors are present in the platelets of blood plasma: platelet-derived growth factor (PDGF), transforming growth factor (TGF), and vascular endothelial growth factor (VEGF). These proteins molecules play a role in tissue angiogenesis, by stimulating the healing and growth of new organic structures, when they contact their respective receptors31,32. Li et al.32 investigated the effects of PRP on human hair follicle growth in vitro and in vivo and explored the possible mechanisms involved. Their report suggested that PRP has the following effects: 1, induces the proliferation of dermal papilla (DP) cells; 2, increases Bcl-2 protein levels, and the expression of this protein during the hair cycle suggests that DP cells are normally protected from apoptosis; 3, increases cell growth and prolongs the survival of hair follicles by activating ERK and Akt signaling, respectively; and 4, may prolong the anagen phase of the hair cycle and stimulate hair growth through the marked increase in the expression of fibroblast growth factor-7 (FGF-7) in DP cells. The final conclusion of the authors was that PRP increases hair growth and hair follicle survival in mice because of its ability to promote cell proliferation and its anti-apoptotic properties.
Growth factors function in the bulge area, where stem cells are present, and they interact with cells of the matrix, thus activating the proliferative phase of the hair. Stem cells, which are primitive and of ectodermal origin, are the source of epidermal cells and sebaceous glands. The germinative cells of the matrix are found at the DP and are of mesenchymal origin. These cells are dependent upon each other, and their simultaneous actions via various growth factors (PDGF, TGF-ß, and VDGF) result in the development of the future follicular unit, which consists of the hair shaft, sebaceous glands, erectus pilus muscle, and the perifolliculum. Although the action of growth factors on the germinative hair cycle has been studied in both the embryologic and adult phases, it has not yet been studied in hair micrograft implantation surgery31. After approval from the local ethics committee, the author established a protocol to verify the efficacy of these factors in the growth and density of implanted follicular units. The study included 20 patients aged 22 to 54 years with male pattern baldness in whom 2 symmetrical areas of 2.5 cm2 were delineated and yielded by using the author's standard technique. Follicular units imbibed with platelet plasma growth factors (obtained from the patient's autologous plasma) were implanted on the right side of the patient's head and standard follicular units were implanted as a control on the left side, with an equal number of micrografts in each side. Before surgery, blood was obtained from each patient and was centrifuged, leaving the platelet-rich plasma (Figure 11). The follicular units were placed in this concentrate for 15 minutes. Thereafter, 10% calcium chloride was added in order to produce the plasmatic gel that would seal the growth factors around the micrografts, which were ready for implantation at that point (Figure 12). The paired t-test was used, with the significance level set at 0.05. Patients were evaluated monthly for 7 months, and the yield of follicular units was counted in the outlined areas. A surgeon measured the density with a magnifying glass and 2 assistants recounted the value. Digital imaging (Pro Plus 4.5 image analysis software, Media Cybernetics) was used as a comparative tool for assessing density. A significant difference in the yield of follicular units was observed when comparing the experimental with the control areas of the scalp (P < 0.001). The areas treated with platelet plasma growth factors demonstrated a yield of 18.7 follicular units per cm2, whereas the control areas demonstrated a yield of 16.4 follicular units per cm2, which was an increase in follicular density of 15.1%. Among the patients who underwent the experimental protocol, the increase in density ranged from only 3% to 52%. In conclusion, this study showed that the areas treated by platelet-rich plasma growth factors had a 15.1% greater hair yield with regard to follicular units and density (Figures 13 to 15). This new development for hair transplant surgery demonstrates that the use of autologous platelet growth factors can improve capillary density with low cost and low morbidity by using a simple technique, which is a significant improvement over conventional techniques. Thus, this technique is an important contribution to implantation surgery, especially for patients with low hair density and very thin hair in the donor area. Although these results are significant, further evaluation such as a doubleblind test should be performed to evaluate the final results with outside assistance in asymmetrical areas.
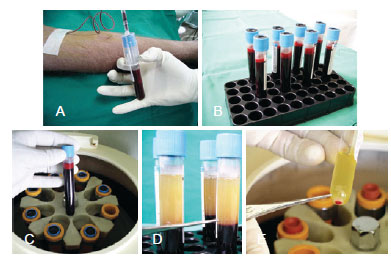
Figure 11 - Preparation of the platelet rich plasma (PRP). In A and B, collecting 80 cc of blood prior the procedure. In C e D, centrifuging the blood. In E, 2 cc from the plasma bottom layer is collected and preserved in a cub.
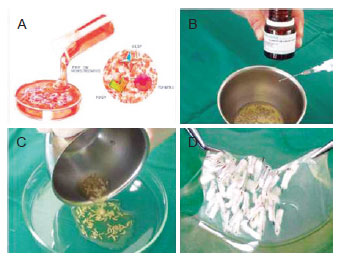
Figure 12 - In A, scheme of growth factors contained in the platelets of the blood plasma: growth factors platelet-derived (PDGF), transforming growth factor (TGF) and vascular endothelial growth factor (VEGF). In B, activation of platelet-rich plasma (PRP) with calcium chloride 10%, transforming fibrinogen to fibrin. In C and D, maintenance of follicular units embedded in gel that releases growth factors for 15 minutes.
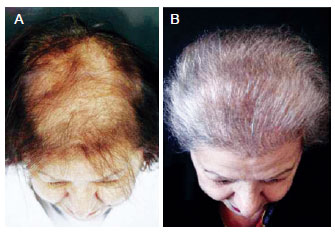
Figure 13 - A 82-year-old patient with hair loss underwent a follicular units and growth factors platelet-derived implant. In A, preoperative aspect. In B, 14 months postoperatively aspect.
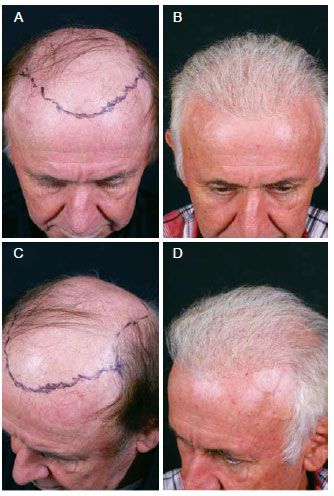
Figure 14 - A 69-year-old patient with hair loss underwent implant follicular units with the use ofplatelet growth factors to activate stem cells. In A and C, preoperative marking. In B and D, postoperative aspect.
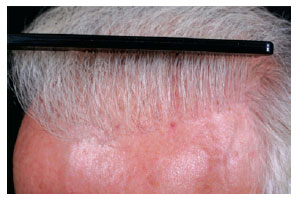
Figure 15 - Detail view of the same patient of Figure 14, 2 years after transplantation.
CONCLUSION
The quality and strength of the implanted hair persists for an indefinite period in some patients because of 1 of the following particular characteristics: high histological quality of the donor area, heredity, hormones, or aging.
Results of hair transplant surgery demonstrate that the use of autologous platelet growth factors can improve capillary density. This offers a new perspective on hair transplantation and is an important contribution to implantation surgery with follicular unit megasessions.
REFERENCES
1. Orentreich N. Autografts in alopecia and other selected dermatologic conditions. Ann N Y Acad Sci. 1959;83:463-79.
2. Juri J. Use of parieto-occipital flaps in the surgical treatment of baldness. Plast Reconstr Surg. 1975;55(4):456-60.
3. Chajchir A, Benzaquen I. Surgical treatment for baldness. Aesthetic Plast Surg. 1982;6(1):33-42.
4. Uebel CO. Baldness surgery: reduction versus flap. Handchir Mikrochir Plast Chir. 1983;15(4):250-3.
5. Nordström REA. "Micrografts" for the improvement of the frontal hairline after hair transplantation. Aesthetic Plast Surg. 1981;5:97-101.
6. Marritt E. Single-hair transplantation for hairline refinement: a practical solution. J Dermatol Surg Oncol. 1984;10(12):962-6.
7. Uebel CO. Punctiform technique with micrografts: a new method for pattern baldness surgery. Presented at Jornada Carioca de Cirurgia Plástica; Rio de Janeiro; August 1986.
8. Uebel CO. Micrograft: a new approach for pattern baldness surgery. Transactions of the International Society of Aesthetic Plastic Surgery, Tenth International Congress; Zurich; September 1989.
9. Uebel CO. Micrografts and minigrafts: a new approach for baldness surgery. Ann Plast Surg. 1991;27(5):476-87.
10. Uebel CO. The use of erbium Yag laser in the capillar microtransplant surgery. In: Badin AZ, ed. Rejuvenescimento facial a laser. Rio de Janeiro: Revinter;1997. p.350-72.
11. Uebel CO. Microtransplante da unidade folicular e a utilização do laser erbium Yag na cirurgia da calvície. In: Horibe EK, ed. Estética clínica e cirúrgica. Rio de Janeiro: Revinter;1999. p.207-15.
12. Uebel CO. Microhaartransplantation: die punktier-technik. In: Lemperle G, ed. Ästhetische chirurgie. Landsburg: Ecomed;1999. p.1-8.
13. Uebel CO. Refining hair restoration technique. Aesthet Surg J. 2002;22(2):181-3.
14. Uebel CO. Hair restoration: micrografts and flaps. São Paulo: OESP Gráfica; 2001.
15. Griffin El. The treatment of female pattern alopecia by hair transplantation. In: Stough DB, Haber RS, eds. Hair replacement: surgical and medical. Philadelphia: Mosby; 1996. p. 210-6.
16. Norwood O. Alopecia: classification and incidence. In: Stough DB, Haber RS, eds. Hair replacement: surgical and medical. Philadelphia: Mosby; 1996. p. 13-9.
17. Uebel CO. Baldness surgery: the mega-punctiform technique. Plast Surg Techniques. 1995;1(2):95-103.
18. Basto FT, Lemos P. Irregular and sinuous anterior hairline in the capillary micrograft. Rev Soc Bras Cir Plast Estet Reconstr. 1996;11(2):15-22.
19. Uebel CO. Improvement of the frontal hairline with the angular flap and micrografts. Transaction Intl Adv Hair Replacem Symposium AAFPRS. Birmingham, AL, 1982.
20. Radwanski HN, Almeida MWR, Aguiar LFS, Altenhofen MS, Pitanguy I. Algoritmo para as alopecias cicatriciais e suas opções de tratamento. Rev Bras Cir Plást. 2009;24(2):170-5.
21. Basto Júnior FT. Calvície feminina: classificação proposta. Rev Bras Cir Plást. 2006;21(4):196-202.
22. Leão CEG, Miranda ES, Rodrigues FHC. Conduta pessoal em cirurgia da calvície. Rev Bras Cir Plást. 2008;23(1):61-6.
23. Basto Júnior FT. Decúbito dorsal: segurança do paciente aliada à sobre-vida das unidades foliculares no transplante capilar. Rev Bras Cir Plást. 2008;23(4):332-6.
24. Muricy JC, Muricy MA. Transplante capilar: incisão sagital versus coronal. Rev Bras Cir Plást. 2006;21(2):102-7.
25. Vendramin FS, Franco D, Franco TR. Método de obtenção do gel de plasma rico em plaquetas autólogo. Rev Bras Cir Plást. 2009;24(2):212-8.
26. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17(2):212-9.
27. Vendramin FS, Franco D, Franco TR. Utilização do plasma rico em plaquetas autólogo nas cirurgias de enxertos cutâneos em feridas crônicas. Rev Bras Cir Plást. 2010;25(4):589-94.
28. Vendramin FS, Franco D, Schamall RF, Franco TR. Utilização do plasma rico em plaquetas (PRP) autólogo em enxertos cutâneos em coelhos. Rev Bras Cir Plást. 2010;25(1):4-10.
29. Almeida ARH, Menezes JA, Araújo GKM, Mafra AVC. Utilização de plasma rico em plaquetas, plasma pobre em plaquetas e enxerto de gordura em ritidoplastias: análise de casos clínicos. Rev Bras Cir Plást. 2008;23(2):82-8.
30. Takikawa M, Nakamura S, Nakamura S, Ishirara M, Kishimoto S, Sasaki K, et al. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011;37(12):1721-9.
31. Uebel CO, Silva JB, Cantarelli D, Martins P. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118(6):1458-66.
32. Li ZJ, Choi HI, Choi DK, Sohn KC, Im M, Seo YJ, et al. Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38(7 Pt 1):1040-6.
33. Sohn KC, Shi G, Jang S, Choi DK, Lee Y, Yoon TJ, et al. Pitx2, a beta-catenin-regulated transcription factor, regulates the differentiation of outer root sheath cells cultured in vitro. J Dermatol Sci. 2009;54(1):6-11.
1. Plastic surgeon, PhD, associate professor of the Division of Plastic Surgery of the Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), full member of the Sociedade Brasileira de Cirurgia Plástica/Brazilian Society of Plastic Surgery (SBCP), Porto Alegre, RS, Brasil
2. Plastic surgeon, PhD, chief of the Division of Plastic Surgery of the PUCRS, full member of the SBCP, Porto Alegre, RS, Brasil
3. Associate plastic surgeon of the Uebel Clinic, aspiring member of the SBCP, Porto Alegre, RS, Brasil
4. Resident physician of the Division Plastic Surgery of the PUCRS, specialist in Hand Surgery and Reconstructive Microsurgery, Porto Alegre, RS, Brasil
Correspondence to:
Carlos Oscar Uebel
Rua Vitor Hugo, 78
Porto Alegre, RS, Brazil - CEP 90630-070
E-mail: carlos@uebel.com.br
Article submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: June 7, 2012
Article accepted: September 2, 2012
This study was performed at Plastic Surgery Department of the Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter