

Original Article - Year 2013 - Volume 28 -
Salvage breast reconstruction: the importance of myocutaneous flaps
Reconstrução mamária de resgate: a importância dos retalhos miocutâneos
ABSTRACT
INTRODUCTION: Breast reconstruction can present an unsatisfactory aesthetic result or complications that could compromise the final result. In such cases, surgeons can perform salvage breast reconstruction, which is defined as a complete revision of a previous reconstruction in the case of failure or an unsatisfactory result from the first reconstruction. This study aims to report the authors' experience in performing salvage breast reconstruction after mastectomy for breast cancer.
METHODS: This was a retrospective study of medical records of patients who underwent salvage breast reconstruction from March 2002 to March 2012.
RESULTS: We identified 57 cases of salvage breast reconstruction. Twenty initial surgeries were performed with prostheses, 16 with transverse rectus abdominis myocutaneous flaps (TRAMs), 11 with expanders, 4 with conservative methods, and 6 with myocutaneous latissimus dorsi muscle flaps (LDMFs). The main cause of reconstruction failure was aesthetic, followed by necrosis, capsular contracture, and implant infection and/or exposure. Salvage reconstruction was performed using LDMF in 27 patients (P < 0.0001), TRAM in 16, and alloplastic material in 14 patients. In 57.9% of cases, the surgeon who performed the salvage reconstruction did not perform the initial reconstruction.
CONCLUSIONS: Most surgeries that had unsatisfactory results, mainly because of poor aesthetics, were performed using alloplastic materials. Salvage reconstructions were performed primarily using myocutaneous flaps by professionals other than those who performed the initial surgery. These flaps have good applicability in salvage reconstructions because they provide healthy and well-vascularized tissue in a previously operated area.
Keywords: Mammaplasty/complications. Breast/surgery. Breast neoplasms. Surgical flaps.
RESUMO
INTRODUÇÃO: A reconstrução mamária pode apresentar um resultado estético insatisfatório ou complicações que comprometam o resultado final. Nesses casos, pode-se realizar a reconstrução mamária de resgate, que é definida como uma revisão completa de uma reconstrução prévia, em caso de resultado insatisfatório ou falha da primeira reconstrução. Este trabalho tem como objetivo reportar a experiência dos autores na realização da reconstrução mamária de resgate pós-mastectomia por câncer de mama.
MÉTODO: Estudo retrospectivo de prontuários de pacientes submetidas a reconstrução mamária de resgate, no período de março de 2002 a março de 2012.
RESULTADOS: Foram identificados 57 casos de reconstrução mamária de resgate. Com relação à cirurgia inicial, 20 foram realizadas com próteses, 16 com retalho miocutâneo do músculo reto abdominal (TRAM), 11 com expansores, 6 cirurgias conservadoras e 4 com retalho miocutâneo do músculo grande dorsal (RGD). A principal causa de falha das reconstruções foi por motivos estéticos, seguida de necrose, contratura capsular e infecção e/ou exposição de implantes. A reconstrução de resgate foi realizada em 27 pacientes com emprego de RGD (P < 0,0001), em 16, com TRAM, e em 14, com material aloplástico. Em 57,9% dos casos, o cirurgião que realizou a reconstrução de resgate não foi o cirurgião da reconstrução inicial.
CONCLUSÕES: A maioria das cirurgias que apresentaram resultados insatisfatórios foi realizada com materiais aloplásticos, sendo a principal causa o aspecto estético deficiente. As reconstruções de resgate foram realizadas principalmente com retalhos miocutâneos e por profissionais diferentes da primeira cirurgia. Os retalhos miocutâneos apresentam boa aplicabilidade nas reconstruções de resgate, por fornecerem tecido sadio e bem vascularizado a uma área manipulada previamente.
Palavras-chave: Mamoplastia/complicações. Mama/cirurgia. Neoplasias da mama. Retalhos cirúrgicos.
Breast cancer affects women worldwide1. Mastectomy, which is often used in the treatment of malignant breast cancer, can be a lifesaving procedure. However, the loss of a breast can cause psychological and psychosocial trauma. Therefore, breast reconstruction has become an important step in post-mastectomy recovery since it can help patients regain a sense of femininity2.
Several breast reconstruction techniques are available, including the use of local flaps, such as the plug flap3 and mammoplasty, lateral flaps such as the thoracodorsal flap4, alloplastic materials (tissue expanders and implants), and numerous autologous flaps5, including microsurgical flaps or even combined techniques. No procedure is better than another in any aspect; however, patients benefit when an informed choice is made based on the surgeon's knowledge, the patient's willingness, and the indications and contraindications of each method5.
These procedures are considered safe but can result in complications (immediate or delayed), unsatisfactory results, compromised surgical margins in the first procedure, or tumor recurrence, indicating failure of the initial breast reconstruction. Complications are more prevalent in patients with risk factors, such as those who have undergone radiotherapy, and are associated with higher rates of capsular contracture, loss of flap volume, and fat necrosis5.
Furthermore, some professionals perform these specialty procedures, including breast reconstructions, without appropriate training6. Therefore, there has been an increase in the number of patients who have undergone poorly indicated techniques and have experienced unsatisfactory and even disastrous results.
To correct breast reconstruction failure and help recover a patient's self-esteem, a surgeon can perform a salvage breast reconstruction. This is defined as a complete revision of a previous reconstruction in the case of unsatisfactory results or failure of primary breast reconstruction7.
The aim of this study is to report the authors' experience in performing salvage breast reconstruction after mastectomy because of breast cancer.
METHODS
We reviewed the medical records of patients who underwent breast reconstruction after mastectomy for breast cancer from March 2002 to March 2012.
The study included patients who had undergone some form of breast reconstruction after mastectomy for breast cancer and required reconstruction for aesthetic reasons, their own preference, complications, or tumor recurrence. We excluded patients who underwent small scar corrections, grafting, or local or regional flap application since they did not require a full review of the first surgery and did not require salvage breast reconstruction.
The following data were collected: age, date of first surgery, type of breast reconstruction performed during the first surgery, risk factors (comorbidities, smoking, obesity, and radio- or chemotherapy), causes of failure of the first procedure (aesthetic issue, patient preference, implant infection/exposure, capsular contracture, necrosis, tumor recurrence, and other), type of salvage reconstruction performed, date of salvage reconstruction, and whether the same surgical team that performed the initial procedure also performed the salvage reconstruction.
The data were assessed using the D'Agostino-Pearson or Shapiro-Wilk test to verify the distribution type. Normally distributed data are presented as mean ± standard deviation. In this case, to compare groups, we used analysis of variance (ANOVA) followed by the Tukey test and/or chi-square test. Freely distributed data are presented as median (25th percentile and 75th percentile), and intergroup comparisons were performed using the Kruskal-Wallis test followed by Dunn's test. In all statistical tests, P values < 0.05 were considered significant. Correlation analysis was performed using the nonparametric Spearman r test (r = correlation). Positive r values indicated a positive correlation between the variables and the r values according to the following scale: 0-0.3, weak; 0.3-0.6, regular; 0.6-0.9, strong; and 0.9-1.0, very strong. Analyses were performed using the BioEstat 5.0 program.
RESULTS
From March 2002 to March 2012, of 1,158 patients who underwent breast reconstruction after mastectomy for the treatment of breast cancer, 55 underwent salvage breast reconstruction and 2 underwent 2 salvage procedures for a total of 57 (4.92%) cases. The average patient age at the time of the new reconstruction was 51.03 ± 10.13 years.
Among the initial failed surgeries, 20 (35.09%) were performed with prostheses (P = 0.035), 16 (28.08%) with transverse rectus abdominis myocutaneous flaps (TRAMs), 11 (19.30%) with tissue expanders (temporary or permanent), 6 (10.52%) with conservative techniques, and 4 (7.01%) with myocutaneous latissimus dorsi muscle flaps (LDMFs) associated with the implant. Forty-five reconstructions were unilateral and 12 were bilateral (Figure 1).
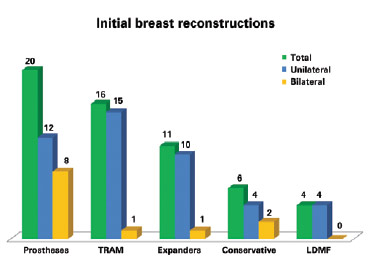
Figure 1 - Breast reconstructions that required salvage. TRAM = transverse rectus abdominis myocutaneous flap; LDMF = latissimus dorsi myocutaneous flap.
The presence of risk factors for complications was observed in 51 (89.50%) patients. The most prevalent factor was chemotherapy in 40 (70.18%) cases, followed by radiotherapy in 32 (56.14%) cases, comorbidities such as hypertension, coronary artery disease, hyper/hypothyroidism, and diabetes mellitus in 15 (26.32%) cases, smoking in 9 (15.80%), and obesity in 4 (7.01%) cases (Figure 2).
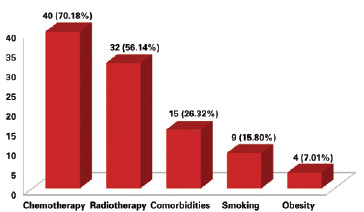
Figure 2 - Number of patients according to the presence of a risk factor for complications.
The main causes of reconstruction failure were aesthetic reasons and patient preference (19 cases), followed by necrosis (17 cases [10 cases of skin necrosis of the flap and 7 cases of steatonecrosis]), capsular contracture (9 cases), implant infection and/or exposure (9 cases), need for expansion of surgical margins due to tumor involvement (2 cases), and tumor recurrence (1 case), but there was no statistically significant difference between them (P = 0.8304) (Figures 3 and 4).
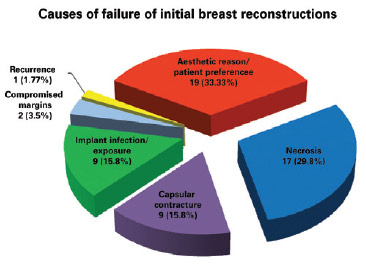
Figure 3 - Causes of initial breast reconstruction failure.
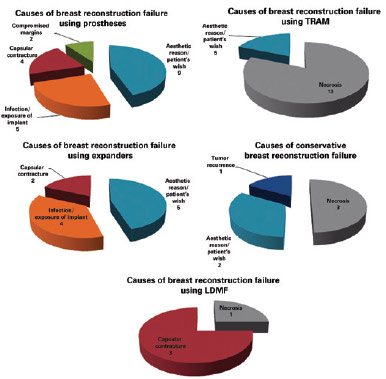
Figure 4 - Causes of initial breast reconstruction failure by technique used. LDMF = latissimus dorsi myocutaneous flap; TRAM = transverse rectus abdominis myocutaneous flap.
Salvage reconstruction was performed in 27 (47.37%) LDMF cases associated with implants (P < 0.0001), 16 (28.08%) with TRAM, 9 (15.77%) with tissue expanders, and 5 (8.78%) with prostheses (Figures 5-16). All 12 initial bilateral reconstructions were salvaged bilaterally; the 3 that were initially unilateral were salvaged bilaterally due to tumor relapse in the other breast (n = 2) or as a prophylactic measure (n = 1). The new intervention was performed at an average of 24 months (12; 40) after the initial surgery. In 57.9% of cases (n = 33), the surgeon who performed the salvage breast reconstruction was not the surgeon who performed the initial reconstruction (P = 0.500).
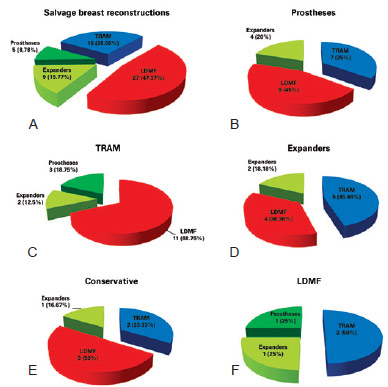
Figure 5 - In A, techniques used in salvage breast reconstructions. In B, C, D, E and F, salvage breast reconstruction techniques used according to initial breast reconstruction. LDMF = latissimus dorsi myocutaneous flap; TRAM = transverse rectus abdominis myocutaneous flap.
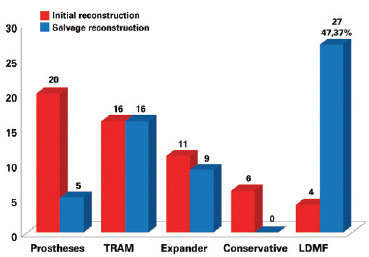
Figure 6 - Number of patients who underwent breast reconstruction according to initial breast reconstruction and salvage breast reconstruction techniques. LDMF = latissimus dorsi myocutaneous flap; TRAM = transverse rectus abdominis myocutaneous flap.
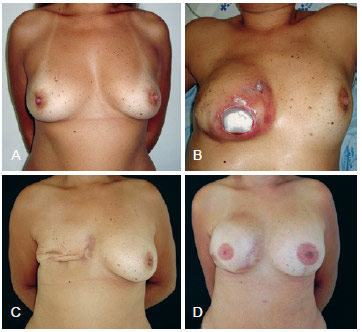
Figure 7 - In A, preoperative aspect. In B, appearance 8 months after right mastectomy with expander placement showing implant extrusion. In C, postoperative implant removal. In D, postoperative aspect after salvage breast reconstruction using a tissue expander and contralateral symmetrization.
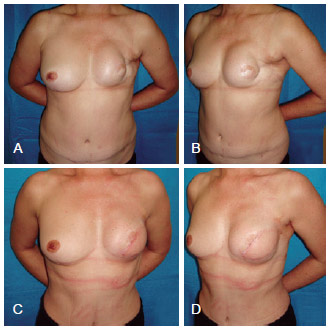
Figure 8 - In A and B, postoperative appearance of left mastectomy and reconstruction using TRAM that progressed to necrosis. In C and D, postoperative aspect after salvage breast reconstruction using LDMF and a prosthesis. LDMF = latissimus dorsi myocutaneous flap; TRAM = transverse rectus abdominis myocutaneous flap.
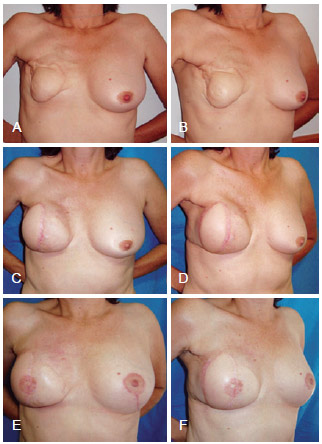
Figure 9 - In A and B, postoperative appearance of right mastectomy with reconstruction using TRAM that progressed to necrosis. In C and D, postoperative appearance of salvage breast reconstruction using LDMF and a prosthesis. In E and F, postoperative appearance of the reconstructed nipple areolar complex and contralateral symmetrization. LDMF = latissimus dorsi myocutaneous flap; TRAM = transverse rectus abdominis myocutaneous flap.
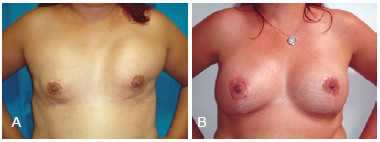
Figure 10 - In A, bilateral breast reconstruction using implants that had an unsatisfactory aesthetic result (implant displacement). In B, bilateral salvage breast reconstruction using tissue expanders.
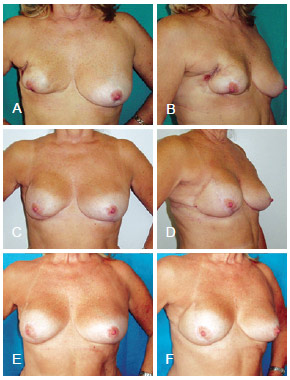
Figure 11 - In A and B, postoperative appearance of right mastectomy and reconstruction using a conservative technique that resulted in necrosis. In C, D, E and F, postoperative aspect of salvage breast reconstruction using LDMF and a prosthesis. LDMF = latissimus dorsi myocutaneous flap.
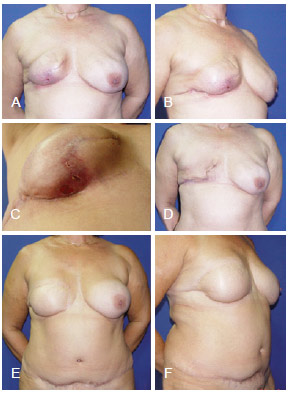
Figure 12 - In A, B and C, appearance 4 months after right mastectomy and reconstruction using a tissue expander that resulted in infection. In D, aspect after tissue expander removal. E and F, postoperative aspect after salvage reconstruction using TRAM. TRAM = transverse rectus abdominis myocutaneous flap.
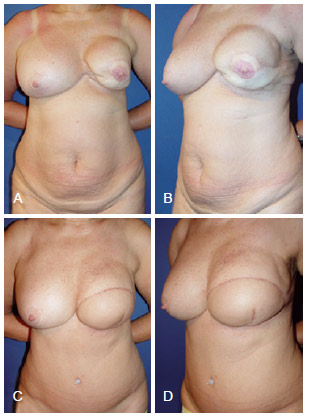
Figure 13 - In A and B, appearance 2 years after left mastectomy and reconstruction using LDMF and a prosthesis that resulted in capsular contracture. In C and D, 7 months after salvage breast reconstruction using TRAM. LDMF = latissimus dorsi myocutaneous flap; TRAM = transverse rectus abdominis myocutaneous flap.
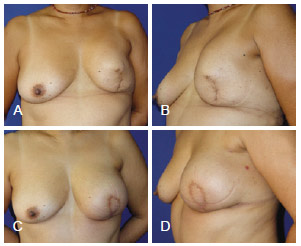
Figure 14 - In A and B, appearance 18 months after left mastectomy and reconstruction using a prosthesis that had unsatisfactory results (the patient wanted more symmetrical breasts without surgery on the right breast). In C and D, appearance 3 months after salvage breast reconstruction using TRAM. TRAM = transverse rectus abdominis myocutaneous flap.
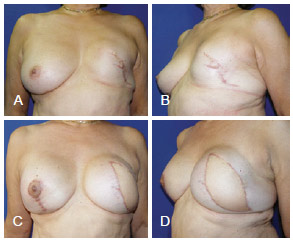
Figure 15 - In A and B, appearance 3 years after left mastectomy and reconstruction using a prosthesis that had unsatisfactory results. In C and D, appearance 3 months after salvage breast reconstruction using LDMF and bilateral prostheses. LDMF = latissimus dorsi myocutaneous flap.
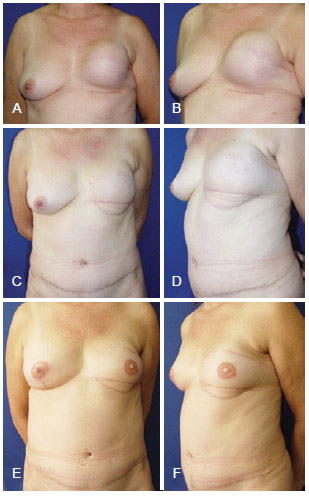
Figure 16 - In A and B, appearance 6 months after left mastectomy, and reconstruction using a prosthesis that had unsatisfactory results. In C and D, appearance 9 months after salvage breast reconstruction using TRAM. In E and F, appearance 1 year and 2 months after salvage reconstruction with a reconstructed nipple areolar complex and a symmetrized contralateral breast. TRAM = transverse rectus abdominis myocutaneous flap.
DISCUSSION
With the gradual increase in breast cancer cases over the past few decades, the demand for post-mastectomy breast reconstruction, either immediate or delayed, has also increased. This increase and patients' increased knowledge of the procedures has led to a change in expectations. These procedures are no longer considered only reconstructive procedures but also aesthetic procedures that demand more harmonious results7.
Delayed or immediate breast reconstruction can be performed with autologous tissues, implants, or a combination of the two. Unfortunately, not all reconstructions exhibit satisfactory results despite the use of implants or flaps8. Breast reconstruction surgery generally has a complication rate of 15-45%, and complications are more prevalent in patients with risk factors9. Reconstructions performed using implants have a complication rate > 40%, and this rate reaches 70% after radiotherapy10. Capsular contracture and infections are the most common complications7.
Few studies have addressed the issue of reconstruction after initial failure. Accordingly, the nomenclature (salvage, secondary, or tertiary) is not clearly standardized. Hamdi et al.7 defined primary breast reconstructions as those performed at the same time as the mastectomy; secondary breast reconstructions are the additional procedures and symmetrization that are carried out after mastectomy; and tertiary, or salvage breast reconstruction, is reconstruction due to failure of the initial surgery.
Hamdi et al.7 examined cases of salvage reconstruction after implant failure and found that the reconstruction rate was 7.8% (54 of 688 patients). In our series, we analyzed all reconstruction types, not just implants, and noted a rate of 4.92% (57 of 1,158 patients). Those authors7 found that the main reason for salvage reconstruction was aesthetic, just as in the present study (Figure 3). One can attribute this finding to the change in the characteristics of patients with breast cancer, such as cohorts consisting of younger patients with greater aesthetic expectations7.
In our series of patients, 51 (89.5%) patients presented with at least one risk factor for complications. Chemotherapy was the most frequent factor (Figure 2). Although not clearly defined as a risk factor11, chemotherapy is believed to impair cellular functions required for postoperative recovery, leading to unfavorable results12. Infection and implant exposure occurred in 9 cases. The infection rate in implant reconstructions can reach 35.4%13, while the exposure rate is 0.25-8.3%14,15.
Necrosis of the reconstructed breast was the second highest cause of intervention. Ofthe 17 patients with necrosis, 13 (76.47%) underwent reconstruction using TRAM. In TRAM, the necrosis rate is 8.2-26.9%16,17, and radiotherapy may lead to higher rates of fat necrosis5. In addition, higher levels of radiation favors capsular contracture18, a complication that was observed in 9 patients in this series, of whom 7 had undergone radiotherapy (r = 0.9999 and P = 0.1582).
Breast reconstruction is a complex procedure, and salvage breast reconstruction in particular is a challenging technique. The sequelae of previous surgeries that are occasionally associated with radiation damage do not favor breast reconstruction with implants in isolation7. In these cases, restoration of locoregional anatomy and removal of scar tissue are important points, and reconstruction with myocutaneous flaps is highly recommended - except in cases with contraindications - since it provides well-vascularized healthy tissue as well as breast volume. In the present series, the initial surgery was performed in most cases with the exclusive use of implants, whereas in salvage surgeries, the use of myocutaneous flaps was prevalent (P = 0.0139), and the use of LDMF was associated with the use of implants, the most frequent technique (P < 0.0001) (Figure 17).
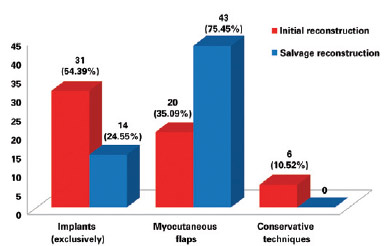
Figure 17 - Number of patients who underwent breast reconstruction in accordance with the use of implants (exclusively), myocutaneous flaps, or conservative techniques in the initial breast reconstruction and salvage breast reconstruction.
Despite the good applicability of myocutaneous flaps, in cases in which there is no tissue loss or radiotherapy damage (and, therefore, good tissue coverage), reconstruction can be performed using alloplastic material (Figure 7). More than half of the patients in the present study (57.9%) were initially operated on by other surgeons, including professionals from other specialties (breast care and oncology). This finding may reflect a deficient physician-patient relationship or even the physician's lack of knowledge about the need for reconstruction, leading patients to seek the advice of other professionals.
CONCLUSIONS
In the series studied, most surgeries failed because of a deficient aesthetic result and were performed using alloplastic material (prostheses and tissue expanders). For salvage reconstructions, myocutaneous flaps were primarily used, and most of the new interventions were performed by professionals other than those who performed the first surgery.
Myocutaneous flaps have good applicability in salvage reconstructions since they provide healthy and well-vasculaized tissue to previously operated areas, restore the anatomy, and provide volume to the reconstructed breast.
Breast reconstruction should be recommended after careful analysis. Plastic surgeons should be aware of the risks of complications that can lead to reconstruction failure and be prepared with new treatment alternatives. Furthermore, the surgeon must explain to the patient the possibility of failure and the need for a new procedure. A good physician - patient relationship must be created to enable the same surgeon to conduct any necessary corrections.
REFERENCES
1. Instituto Nacional de Câncer (Brasil). Estimativa 2012: incidência de câncer no Brasil. Rio de Janeiro: INCA; 2011. 118p.
2. Colakoglu S, Khansa I, Curtis MS, Yueh JH, Ogunleye A, Haewyon C, et al. Impact of complications on patient satisfaction in breast reconstruction. Plast Reconstr Surg. 2011;127(4):1428-36.
3. Daher JC. Breast island flaps. Ann Plast Surg. 1993;30(3):217-23.
4. Holmström H, Lossing C. The lateral thoracodorsal flap in breast reconstruction. Plast Reconstr Surg. 1986;77(6):933-43.
5. Serletti JM, Fosnot J, Nelson JA, Disa JJ, Bucky LP. Breast reconstruction after breast cancer. Plast Reconstr Surg. 2011;127(6):124e-35e.
6. Goldenberg D. A preocupação com as novas gerações. Rev Bras Cir Plást. 2011;26(2):187.
7. Hamdi M, Casaer B, Andrades P, Thiessen F, Dancey A, D'Arpa S, et al. Salvage (tertiary) breast reconstruction after implant failure. J Plast Reconstr Aesthet Surg. 2011;64(3):353-9.
8. Losken A, Carlson GW, Schoemann MB, Jones GE, Culbertson JH, Hester TR. Factors that influence the completion of breast reconstruction. Ann Plast Surg. 2004;52(3):258-61.
9. Cosac OM, Costa LA, Barros APGSH. Reconstrução mamária bilateral com retalhos pediculados. In: Melega JM, Viterbo F, Mendes FH, eds. Cirurgia plástica: os princípios e a atualidade. Rio de Janeiro: Guanabara Koogan; 2011.p.732-42.
10. Dickson MG, Sharpe DT. The complications oftissue expansion in breast reconstruction: a review of75 cases. Br J Plast Surg. 1987;40(6):629-35.
11. Warren Peled A, Itakura K, Foster RD, Hamolsky D, Tanaka J, Ewing C, et al. Impact of chemotherapy on postoperative complications after mastectomy and immediate breast reconstruction. Arch Surg. 2010;145(9):880-5.
12. Oh E, Chim H, Soltanian HT. The effects of neoadjuvant and adjuvant chemotherapy on the surgical outcomes of breast reconstruction. J Plast Reconstr Aesthet Surg. 2012;65(10):e267-80.
13. Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: two year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109(7):2265-74.
14. Disa JJ, Ad-El DD, Cohen SM, Cordeiro PG, Hidalgo DA. The premature removal of tissue expanders in breast reconstruction. Plast Reconstr Surg. 1999;104(6):1662-5.
15. Pusic AL, Cordeiro PG. An accelerated approach to tissue expander for breast reconstruction: experience with intraoperative and rapid postoperative expansion in 370 reconstructions. Plast Reconstr Surg. 2003;111(6):1871-5.
16. Kroll SS. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106(3):576-83.
17. Watterson PA, Bostwick J 3rd, Hester TR Jr, Bried JT, Taylor GI. TRAM flap anatomy correlated with a 10-year clinical experience with 556 patients. Plast Reconstr Surg. 1995;95(7):1185-94.
18. Behranwala KA, Dua RS, Ross GM, Ward A, A'hern R, Gui GP. The influence of radiotherapy on capsule formation and aesthetic outcome after immediate breast reconstruction using biodimensional anatomical expander implants. J Plast Reconstr Aesthet Surg. 2006;59(10):1043-51.
1. Plastic Surgeon, full member of the Sociedade Brasileira de Cirurgia Plástica/Brazilian Society of Plastic Surgery (SBCP), Head of the Plastic Surgery Service, Hospital das Forças Armadas, Brasília, DF, Brazil
2. Resident Physician of the Plastic Surgery Service, Daher Lago Sul Hospital, Brasília, DF, Brazil
3. Plastic Surgeon, full member of the SBCP, Tutor of the Plastic Surgery Service, Daher Lago Sul Hospital, Brasília, DF, Brazil
4. Plastic Surgeon, full member of the SBCP, Head of the Plastic Surgery, Daher Lago Sul Hospital, Brasília, DF, Brazil
Correspondence to:
Ognev Meireles Cosac
Condomínio Villages Alvorada - cj. 17 - casa 10 - Lago Sul
Brasília, DF Brazil - CEP 71680-351
E-mail: ognev@terra.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article submitted: October 5, 2012
Article accepted: January 7, 2013
This study was performed at the CIRPLAS Clinic, the Dra Marcela Cammarota Clinic, and the Di Lamartine Clinic, Brasília, DF, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter