

Original Article - Year 2013 - Volume 28 -
Influence of aging on the skin quality of white-skinned women: the role of collagen, elastic material density, and vascularization
Influência do envelhecimento na qualidade da pele de mulheres brancas: o papel do colágeno, da densidade de material elástico e da vascularização
ABSTRACT
BACKGROUND: In the present study, we aimed to evaluate the influence of aging on the skin quality of white-skinned women by assessing collagen levels, elastic material density, and vascularization.
METHODS: Histological and morphometric analyses were performed on 218 preauricular skin fragments from white-skinned women who underwent facial cosmetic surgery. Anti-CD34 was used to identify the blood vessels, Weigert's staining was used to visualize elastic fibers, and Picro-sirius Ultra Red staining was employed for analyzing and quantifying the expression of type I, III, and total collagen. Data were analyzed according to the following donor age groups: < 40, 40-49, 50-59, 60-69, and > 70 years.
RESULTS: Fragmentation and disorganization of collagen fibers were observed in certain samples, particularly in samples from patients aged > 60 years. Significant differences between age and the thickness of the dermis and epidermis were not detected. However, a relationship was identified between age and the percentages of type I, III, and total collagen, and an increase of elastic fibers density was associated with age progression (P < 0.001). The comparison of the skin of patients with a decade difference in age did not reveal a significant difference in the elastic material quality; however, when the age difference was of 2 decades or more, there was a significant difference in elastic fibers (P < 0.05). The difference in the number of blood vessels between the groups was not significant (P = 0.112).
CONCLUSIONS: Aging promoted collagen reduction, fiber degradation and fragmentation, and increased disorganized elastic material density; however, it did not affect the number of dermal blood vessels.
Keywords: Skin. Collagen. Skin aging. Blood vessels. Elastic tissue.
RESUMO
INTRODUÇÃO: O objetivo deste estudo é avaliar a influência do envelhecimento na qualidade da pele de mulheres brancas, analisando o colágeno, as fibras elásticas e a vascularização.
MÉTODO: Foi realizada análise histológica e morfométrica de 218 retalhos pré-auriculares de mulheres brancas, que se submeteram a cirurgia estética facial. Foram utilizados o imunomarcador AntiCD 34, que evidencia os vasos sanguíneos, a coloração de Weigert, para visibilização das fibras elásticas, e a coloração de Picrosirius Ultrared, para analisar e quantificar os colágenos I, III e total. Os dados foram analisados de acordo com a faixa etárias das doadoras: < 40 anos, 40 anos a 49 anos, 50 anos a 59 anos, 60 anos a 69 anos, e > 70 anos.
RESULTADOS: Foi observada fragmentação e desorganização das fibras de colágeno, especialmente acima de 60 anos. Não houve diferenças significantes entre a idade e a espessura da derme e da epiderme, porém foi identificada relação com as porcentagens de colágeno I, III e total (P < 0,001). Houve aumento da densidade de fibras elásticas com a progressão da idade (P < 0,001). Comparando-se as peles das pacientes de faixas etárias vizinhas, com diferença de uma década entre elas, não houve diferença significativa na quantidade de material elástico dessas peles; porém, ao se comparar aquelas com diferença de 2 ou mais décadas nas faixas etárias, o aumento foi significante (P < 0,05). A diferença do número de vasos não foi significante (P = 0,112).
CONCLUSÕES: O envelhecimento promoveu redução do colágeno, degradação e fragmentação das fibras, e aumento da densidade de material elástico desorganizado, e não influenciou no número de vasos sanguíneos da derme.
Palavras-chave: Pele. Colágeno. Envelhecimento da pele. Vasos sanguíneos. Tecido elástico.
The average life expectancy in Brazil has increased from 69.3 to 72.7 years (1997-2007), owing to the improvement of the national human development index1. Preliminary results of the 2010 Brazilian Census showed that the number of individuals aged < 25 years was reduced compared to the 2000 Census. Simultaneously, the number individuals in the population aged > 65 years increased from 4.8% in 1991 to 5.9% in 2000 and to 7.4% in 2010. This increase was even higher in southern and southeastern Brazil, where it reached 8.1%2.
Aging is a natural process, and the skin represents an ideal marker of chronological age3. The exposed skin is subject to environmental damage, particularly that caused by ultraviolet radiation (UVR). Therefore, skin aging is classified as intrinsic or chronological, and extrinsic or photoaging4.
We aimed to evaluate the influence of age on the skin quality of white-skinned women by examining preauricular fragments of patients who underwent aesthetic facial plastic surgery. Collagen expression and the density of elastic fibers and blood vessels were analyzed.
METHODS
This study was approvedby the ResearchEthics Committee of the Pontifical Catholic University of Paraná (PUCPR; no. 0153.0.084.000-11) in the Brazilian Committee for Research Ethics (CONEP), and the Research Ethics Committee (CEP; no. 6119), according to opinion nº 0005140/11, in a meeting held on June 29, 2011.
The participants of this study were in ideal physical and mental health and signed an informed consent form.
Information regarding comorbidities, previous disease history and conditions, and lifestyle habits was collected.
Patients who presented factors that could influence skin quality were excluded from the study, including smoking, artificial tanning, topical treatments, botulinum toxin application, dermal filling, and previous surgeries.
Fragments of preauricular skin that were discarded during facial plastic procedures were obtained between June 2009 and May 2011.
These fragments, placed onto filter paper and fixed in 10% formalin, were prepared in the Laboratory of Experimental Anatomical Pathology at PUCPR, and a 1 cm2 sample of the central portion of these fragments was used for histological processing. Blocks were prepared and sliced into 5-µm-thick sections, which were then mounted onto slides.
The slides were stained with hematoxylin and eosin for general and organizational study of the skin and for measuring the epidermal and dermal thickness.
Picro-sirius Ultra Red staining (PSR) was applied to 5 sites of each histological section for evaluating collagen density and organization. The density of collagen in each fragment was calculated based on the mean value of these 5 sites.
The anti-CD34 immunomarker was used for identification and quantification of the blood vessels. The valid fields were analyzed, which ranged between 5-10, depending on the area of the histological section examined. Weigert's staining was used to identify elastic fibers in 10 sites of each skin fragment.
Images were captured with a Sony® CCD101 camera, transmitted to a Sony® Trinitron color monitor, frozen, and scanned using a plate Oculus® TCX. Subsequently, image analysis was performed using Image Plus® 4.5 software for Windows® (Media Cybernetics).
The results of collagen density, elastic fiber density, and the number of blood vessels were expressed as mean, median, minimum values, maximum values, and standard deviations. Pearson›s correlation coefficient was estimated to assess the association between age and collagen density. A one-factor model analysis of variance (ANOVA) was used for comparisons between age groups. Data were analyzed using Statistica v.8.0 software through parametric tests according to the results obtained. ANOVA was performed to evaluate the density and organization of collagen and to compare between the age groups in relation to the percentage of fibers. The least significant difference (LSD) test was used for multiple comparisons. The number of blood vessels between age groups was compared using the Kruskal-Wallis nonparametric test.
A null hypothesis rejection level of P < 0.05 (5%) was used for all tests.
RESULTS
We selected 218 patients from an initial sample of 250 women treated during the study period. Six patients who were smokers, 9 who were ex-smokers, and 17 who presented other confounding factors were excluded. Among the 218 selected participants, 204 were included in the collagen fiber analysis, 203 in the elastic fiber analysis, and 200 in the blood vessel assessment. The differences in these numbers resulted from the exclusion of some cases biased by technical artifacts.
The patient ages ranged from 33 to 77 years, with a mean of 54.9 ± 8.68 years (Table 1). Microscopic analysis of histological sections stained with hematoxylin and eosin showed no significant changes between the age groups. The epidermal and dermal thickness (nm) did not differ significantly between age groups (P = 0.152 and P = 0.506, respectively).

Discrete disorganization and fragmentation of collagen fibers were observed until the sixth decade of life, and were more evident in this age group.
Skin histological sections from women aged < 50 years contained a substantial number of neatly arranged type I collagen fibers. In contrast, skin samples from women aged 50-59 years exhibited discrete collagen fragmentation (Figure 1). In skin samples from women aged 60-69 years, a marked decrease of type I collagen was detected, and the presence of collagen type III fibers was noted. Skin histological sections from women aged 70-79 years exhibited severe disruption and fragmentation of type I collagen, and the presence of type III collagen was evident (Figure 1).

Figure 1 - Skin histological sections depicting progressive collagen disorganization (Picro-sirius Ultra Red - 400×). In A, < 50 years. In B, 50-59 years. In C, 60-69 years. In D, 70-79 years. Red = type I collagen. Green = type III collagen.
A decreased total collagen density was associated with aging (P < 0.001) and with the percentages of types I and III collagen (P < 0.001) (Table 2).
Significant differences (P < 0.001) were found in all pairwise comparisons between age groups in relation to the amount of type I, III, and total collagen, with the exception of the comparison between the 60-69 and > 70 years age groups (P = 0.007 for type I collagen, P = 0.076 for type III collagen, and P = 0.012 for total collagen).
Qualitative analysis of the elastic fibers indicated the following findings in each age group:
< 50 years: the presence of slender normal or slightly thickened fibers in the papillary dermis (Figure 2A);

Figure 2 - Photomicrographs of skin histological sections showing elastic modifications in different age groups (Weigert's stain, 400×). In A, < 40 years. In B, 40-49 years. In C, 50-59 years. In D, 60-69 years. In E, > 70 years. The arrows indicate elastic fibers.
41-50 years: the presence of lysis and alterations of the fibers, which began to appear focally thickened (Figure 2B); 51 -60 years: the fibers were tangled and often fragmented (Figure 2C); 61-69 years: the changes became more evident, with loss of the normal fibrillar structure. The fibers became entangled and formed irregularly thickened masses (Figure 2D); > 70 years: the presence of degenerated fibers with destruction of the elastin network, resulting in agglomeration of amorphous and elastotic material throughout the dermis, with the appearance of disintegration and decomposition (Figure 2E).
The mean percentage of the examined area in histological sections showed a progressive increase in elastic material with aging (P < 0.001) (Table 3). The significant difference between age groups required a pairwise comparison (Table 4).
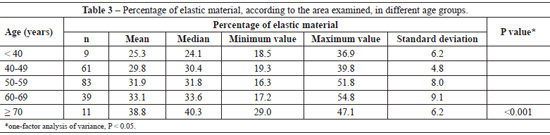

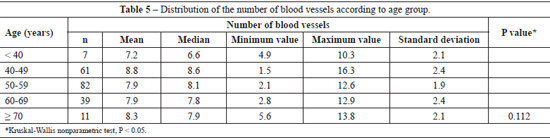
The mean number of dermal vessels was similar (P = 0.112) between the different age groups (Table 5), although they were more irregular and dilated in samples from women aged > 60 years (Figure 3).
DISCUSSION
Due to the increasing elderly population, including that of Brazil, the skin changes resulting from aging has become a matter of great interest.
The search for facial rejuvenation techniques has grown substantially. In the present study, although most of the 218 patients were between 50 and 69 years of age, women at the age extremes of 33 and 77 years underwent the same facial rejuvenation procedures.
Aging is a natural and immutable process for all organs5,6. The skin is an exposed organ, and is therefore subjected to environmental damage such as that caused by UVR, which plays a relevant role in extrinsic aging and photoaging7-13.
Age, skin color, photoexposure, and smoking are independent risk factors for skin aging3. Among the initially evaluated group, 6% of patients were smokers or ex-smokers for more than 5 years according to the World Health Organization criteria14 and were excluded from the analysis.
According to Landau15, the most important aspect of intrinsic aging is the flattening of the dermal-epidermal junction, with a decrease of the contact surface between the dermis and the epidermis. However, epidermal thickness remains constant over time, and the dermal thickness is reduced from the eighth decade of life. The thinning of the epidermis and flattening of the dermal-epidermal junctions might cause cutaneous atrophy6,9. In our sample, there was no significant difference in the epidermal and dermal thickness between the evaluated age groups, in contrast to the observations of Landau15. However, structural and functional alterations of the dermal-epidermal junction have been reported by Le Varlet et al.16.
Oriä et al.17 observed a significant decrease in the epidermal and dermal thickness of patients aged > 60 years, and gradual reduction in the dermal-epidermal contact surface.
The participants of this study were in perfect health, which might have contributed to better skin quality, particularly among the younger age groups.
The main changes visualized in aged skin occurred in the dermal collagen, leading to modifications of its biomechanical properties.5
A mechanism that is responsible for aging is a decrease in the number of proteasomes, which contain multicatalytic enzymes that degrade oxidized and deformed proteins that interfere in the activity of fibroblasts, leading to decreased protein synthesis and increased proteolysis18.
Actinic damage to photoaged skin would be manifested histologically by a dermal inflammatory infiltrate and an increase in elongated and flattened fibroblasts, which are still capable of producing collagen. This suggests that alterations in collagen synthesis would not be the cause of decreases in type I and III collagen.19 Other aging-related skin changes include volume loss; reduction of fibroblasts, mast cells, and blood vessels; shortening of capillary loops; and nervous termination abnormality20. These alterations would lead to decreased synthesis of type I and III collagen, particularly in photoaged skin, thus reducing the adhesion between cells and dermal fibers, with lower levels of mechanical stimulation21. These directional and functional alterations in dermal collagen would be more pronounced from the seventh decade of life22.
Fisher et al.23 demonstrated in vitro that old fibroblasts retain the ability to produce collagen. This characteristic was evident in the present study, when comparing the amounts of type I, III, and total collagen between the age groups. The amount of collagen diminished with aging. As photoexposure is cumulative, the longer the duration of photoexposure, the greater the collagen damage and the alterations of the mechanical properties of the skin23.
In the analysis of the PSR-stained histological sections, we detected that the type I collagen fibers (strongly birefringent and yellowish to reddish) were dominant and very well organized in younger patients. However, these same fibers underwent a gradual transformation in more advanced age groups, presenting a very disorganized appearance in patients aged > 70 years, in which type III collagen fibers (weakly birefringent and greenish) were more evident. Faria et al.6 suggested that the amount of collagen remains the same, and that only modifications in fiber organization occur. In contrast to this previous report, a linear decrease in the percentage of type I, III, and total collagen was observed in the present study, and structural alterations in the direction and organization of the fibers (fragmented appearance) were more pronounced in patients aged 60-69 and > 70 years. The decrease in collagen and its organization, and even the fragmentation of the fibers, might partly explain skin fragility in the elderly.
In the study of El-Domyati et al.24 that evaluated skin samples of 38 donors exposed and not exposed to solar radiation, skin changes started from the fifth decade of life but were only significant in the eighth and ninth decades, when only trace amounts of collagen were noted. It is possible that the number of cases in our sample (n = 218) contributed to the difference in results. The decrease in collagen density, its disorganization, and fragmentation have been reported as the cause of wrinkle development6,9.
In this study, we demonstrated that aging is accompanied by a decrease in collagen density, the causes of which are yet not fully understood. The properties and manner of aging may vary among individuals, according to lifestyle habits, current and past disease history, genetic charges, and environmental influences.
A decreased vascular network might lead to decreased cellular oxygenation in the elderly, with increased levels of reactive oxygen species (ROS) that cause molecular damage and are facilitators of apoptosis and cell death3,16. This situation might be influenced by environmental factors, such as smoking and UVR.
If the fibroblasts of elderly individuals continue to produce collagen, it is likely that the decrease in collagen is a result of its increased degradation, among other factors. Aging is associated with an increase of matrix metalloproteinases (MMPs), which are enzymes responsible for collagen degradation, without equivalent increases of their inhibitors. The beneficial effect of tretinoin on aged skin due to its inhibitory effect on MMPs may be an indicator of this phenomenon5.
Dermal elastic fibers are responsible for the physiological elasticity and resilience of the skin25. The degeneration of these fibers and collagen promotes the reduction of skin elasticity and wrinkle formation26. The density of the elastin network increases from birth until it reaches its final volume, around the age of 20 years in women and 40 years in men27.
According to Bouissou et al.28, chronological aging of elastic fibers and actinic elastosis are 2 distinct processes. Elastotic material resembles elastin biochemically, although it is disorganized and the proportion of several of its constituents is abnormal29. In areas exposed to solar radiation, the progressive destruction of the entire dermal elastin network occurs. Elastic fibers become thickened, tangled, degraded, and dysfunctional28-30.
We analyzed samples of preauricular skin, a region that receives intense photoexposure, and also observed alterations in the elastic fiber quality and an increase in the elastic material density associated with age progression. After the age of 70 years, the elastic fibers were degenerated, thickened, tangled, and often fragmented, resulting in a cluster of amorphous and elastotic material throughout the dermis with a disintegrated and decomposed appearance.
The consequences of alterations in the organization and quality of elastic fibers, despite an apparent increase in their density, include mechanical changes to the skin surface such as wrinkling and flaccidity, particularly on the face and neck29. In the dermis of elderly individuals, there is an increase in elastin density27. According to Braverman & Fonferko3, this increase results from intense collagen loss and elastic fiber thickening, thus causing an increase in the elastic component in the elderly compared to young individuals.
Alterations of microcirculation result in decreased blood flow, resulting in paleness and reduced nutrient exchange. Reduction of capillary loops in elderly skin have been reported, both in photoexposed and photoprotected regions31-33.
According to Smith34, there is a lower number of vessels of all sizes in the elderly, including the capillary loops, as well as wall thinning and alterations in the basal membrane.
In this study, skin samples were analyzed from a region that received intensive photoexposure. We did not observe a significant decrease in the number of vessels, but only ectasia. The sample size of more advanced age groups should be increased in future studies to clarify this issue. Vascular abnormalities associated with aging are usually found only in individuals aged > 70 years35.
Further studies are needed to clarify several important questions that remain unanswered. We will only be able to establish effective methods for the prevention and treatment of aging skin, as well as extrapolate their use to other organs, after achieving an accurate understanding of its process.
CONCLUSIONS
Skin aging leads to qualitative and degenerative alterations in the dermis of white-skinned women, with decreases in type I, III, and total collagen fibers. Fragmentation and disorganization of the collagen fibers were detected, particularly in patients aged > 60 years. An increased density of the elastic material resulted in agglomeration of amorphous material with modification of the quality and organization of elastic fibers. However, aging did not significantly influence the number of blood vessels.
REFERENCES
1. Instituto Brasileiro de Geografia e Estatística. Tábuas completas de mortalidade. Disponível: http://www.ibge.gov.br. Acesso em: 1/12/2009.
2. Instituto Brasileiro de Geografia e Estatística. Disponível em: www.ibge.gov.br/censo2010. Acesso em: 20/S/2010.
3. Suehara LY, Simone K, Maia M. Avaliação do envelhecimento facial relacionado ao tabagismo. An Bras Dermatol. 2006;S1(1):34-9.
4. Mine S, Fortunel NO, Pageon H, Asselineau D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging. PloS One. 200S;3(12):e4066.
5. Moi RC. Envelhecimento do sistema tegumentar: revisão sistemática da literatura [dissertação de mestrado]. Ribeirão Preto: Universidade de São Paulo; 2004.
6. Faria JCM, Tuma Junior P, Costa MP, Quagliano AP, Ferreira MC. Envelhecimento da pele e colágeno. Rev Hosp Clin Fac Med S Paulo. 1995;50(Supl.):39-43.
7. Universidade do Rio de Janeiro. Índice ultravioleta/LEPA/UFRJ. Disponível: http://www.indiceuv.ufrj.br. Acesso em: 20/S/2010.
S. Vicedo Ortega Y, Vicedo Tomey A. Metaloproteinasas de la matriz y envejecimiento cutâneo. Rev Habanera Cienc. Med. 2003;2(5). Disponível em: http://www.ucmb.sld.cu/rhab/index.html. Acesso em: 12/9/2010.
9. Fisher GJ, Kang S, Varani J, Bata-Csorgo, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;13S(11):1462-70.
10. Pagani EA, Jesus AMP, Pereira GC, Neves RG, Nascimento LV. Envelhecimento cutâneo: estudo comparativo clínico, histopatológico e histoquímico de áreas expostas e não expostas à luz solar. An Bras Dermatol. 199S;73(6):523-32.
11. Grönniger E, Weber B, Heil O, Peters N, Stäb F, Wenck H, et al. Aging and chronic sun exposure cause distinct epigenetic changes in human skin. PLoS Genet. 2010;6(5):e1000971.
12. Yaar M, Gilchrest BA. Skin aging: postulated mechanisms and consequent changes in structure and function. Clin Geriatr Med. 2001;17(4):617-30.
13. Röck K, Grandoch M, Majora M, Krutmann J, Fischer JW. Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet B (UVB): new insights into mechanisms of matrix remodeling. J Biol Chem. 2011;2S6(20):1S26S-76.
14. World Health Organization. Tobacco country profiles. 2nd ed. Proceedings of the 12th World Conference on Tobacco or Health. Helsinki: WHO; 2003.
15. Landau M. Exogenous factors in skin aging. Curr Probl Dermatol. 2007;35:1-13.
16. Le Varlet B, Chaudagne C, Saunois A, Barré P, Sauvage C, Berthou-loux B, et al. Age-related functional and structural changes in human dermo-epidermal junction components. J Investig Dermatol Symp Proc. 199S;3(2):172-9.
17. Oriá RB, Ferreira FVA, Santana EN, Fernandes MR, Brito GAC. Estudo das alterações relacionadas com a idade na pele humana utilizando métodos de histo-morfometria e autofluorescência. An Bras Dermatol. 2003;7S(4):425-34.
18. Widmer R, Ziaja I, Grune T. Protein oxidation and degradation during aging: role in skin aging and neurodegeneration. Free Radic Res. 2006;40(12):1259-68.
19. Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoa-ging: mechanisms and repair. J Am Acad Dermatol. 2006;55(1):1-19.
20. Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol. 2007;157(5):874-87.
21. Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblasts function and defective mechanical stimulation. Am J Pathol. 2006;168(6):1861-8.
22. Moragas A, García-Bonafé M, Sans M, Torán N, Huguet P, Martín-Plata C. Image analysis of dermal collagen changes during skin aging. Anal Quant Cytol Histol. 1998;20(6):493-9.
23. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419-28.
24. El-Domyati, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, im-munohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11(5):398-405.
25. Mahoney MG, Brennan D, Starcher B, Faryniarz J, Ramirez J, Parr L, et al. Extracellular matrix in cutaneous ageing: the effects of 0.1% copper-zinc malonate-containing cream on elastin biosynthesis. Exp Dermatol. 2009;18(3):205-11.
26. Miyasaka M, Sakai S, Kusaka A, Endo Y, Kobayashi M, Kobayashi K, et al. Ultrasonic tissue characterization of photodamaged skin by scanning acoustic microscopy. Tokay J Exp Clin Med. 2005;30(4):217-25.
27. Pasquali-Ronchetti I, Baccarani-Contri M. Elastic fiber during development and aging. Microsc Res Tech. 1997;38(4):428-35.
28. Bouissou H, Pieraggi MT, Julian M, Savit T. The elastic tissue of the skin. A comparison of spontaneous and actinic (solar) aging. Int J Dermatol. 1988;27(5):327-35.
29. Wulf HC, Sandby-Moller J, Kobayasi T, Gniadecki R. Skin aging and natural photoprotection. Micron. 2004;35(3):185-91.
30. Braverman IM, Fonferko E. Studies in cutaneous aging: I. The elastic fiber network. J Invest Dermatol. 1982;78(5):434-43.
31. Kelly RI, Pearse R, Bull RH, Leveque JL, Rigal J, Mortimer PS. The effects of aging on the cutaneous microvasculature. J Am Acad Der-matol. 1995;33(5 Pt 1):749-56.
32. Chung JH, Yano K, Lee MK,Youn CS, Seo JY, Kim KH, et al. Differential effects of photoaging vs intrinsic aging on the vascularization of human skin. Arch Dermatol. 2002;138(11):1437-42.
33. Chung JH, Eun HC. Angiogenesis in skin aging and photoaging. J Dermatol. 2007;34(9):593-600.
34. Smith L. Histopathologic characteristics and ultrastructure of aging skin. Cutis. 1989;43(5):414-24.
35. Braverman IM, Yen A. Ultrastructure of the human dermal microcirculation. II. The capillary loops of the dermal papillae. J Invest Dermatol. 1977;68(1):44-52.
1. Master in Surgery, Gynecologist and Obstetrician at the Holy House of Mercy of Ponta Grossa (SCMPG), Ponta Grossa, PR, Brazil.
2. Doctor in Experimental Surgery, Associate Professor of the Department of Surgery, Federal University of Paraná (UFPR), Curitiba, PR, Brazil.
3. Master in Surgery, Dermatologist at the SCMPG, Ponta Grossa, PR, Brazil.
4. Master in Surgery, Plastic Surgeon, full member of the Sociedade Brasileira de Cirurgia Plástica/Brazilian Society of Plastic Surgery (SBCP), Curitiba, PR, Brazil.
5. Plastic Surgeon, full member of SBCP, Curitiba, PR, Brazil.
6. Master in Surgery, Professor of Pathological Anatomy at the State University of Ponta Grossa (UEPG), Ponta Grossa, PR, Brazil.
7. Resident Doctor in General Surgery, Angelina Caron Hospital, Campina Grande do Sul, Paraná, Brazil.
Correspondence to:André Auersvald
Alameda Présidente Taunay, 1.756
Curitiba, PR, Brazil - CEP 82030-590
E-mail: andreauersvald@uol.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: January 6, 2013
Article accepted: February 27, 2013
This study was performed at the Pontifical Catholic University of Paraná (PUCPR), Curitiba, PR, Brazil.




 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter