

Original Article - Year 2013 - Volume 28 -
Protocol for the prevention of venous thromboembolism at the Ivo Pitanguy Institute: efficacy and safety in 1351 patients
Protocolo de prevenção de tromboembolismo venoso no Instituto Ivo Pitanguy: eficácia e segurança em 1.351 pacientes
ABSTRACT
INTRODUCTION: Thromboembolic events are a serious concern due to the high rates of morbidity and mortality as well as the possibility of existing disease presenting with scarce and often nonspecific symptoms. Prevention is the most effective management method for this kind of event, which can quickly lead to death once it occurs.
METHODS: A retrospective study was conducted between May 2009 and May 2010 on patients undergoing plastic surgery at the Ivo Pitanguy Institute. All patients underwent the protocol for the prevention of venous thromboembolism after being assessed for risk factors. These factors were summed to generate a score, which determined the prophylaxis to be implemented.
RESULTS: During one year, 1351 patients were assessed. There was no incidence of venous thromboembolism. There were 16 cases of hematoma, 9 (56.25%) of which occurred after heparin prophylaxis and 7 (43.75%) of which occurred without the use of prophylaxis.
CONCLUSIONS: The protocol for the prevention of venous thromboembolism at the Ivo Pitanguy Institute was effective, with no occurrence of VTE cases and the incidence of hematomas remained below that found in the medical literature.
Keywords: Venous thromboembolism. Venous thrombosis/prevention & control. Plastic surgery.
RESUMO
INTRODUÇÃO: Eventos tromboembólicos causam grande preocupação, em decorrência das altas taxas de morbidade e mortalidade existentes e da possibilidade de apresentação clínica com sintomas escassos e, muitas vezes, inespecíficos. A prevenção é a maneira mais eficaz de lidar com esse tipo de evento, que, uma vez estabelecido, pode levar rapidamente à morte.
MÉTODO: Foi realizado estudo retrospectivo, no período entre maio de 2009 e maio de 2010, com pacientes submetidos a cirurgia plástica no Instituto Ivo Pitanguy. Todos os pacientes foram submetidos ao protocolo de prevenção de tromboembolismo venoso, após serem avaliados quanto aos fatores predisponentes e de risco. A soma desses fatores gerou uma pontuação, que determinou a profilaxia a ser adotada.
RESULTADOS: Foram avaliados 1.351 pacientes durante o período de um ano. Não houve incidência de tromboembolismo venoso. Foram observados 16 casos de hematoma, 9 (56,25%) deles ocorreram após profilaxia com heparina e 7 (43,75%) sem o uso de quimioprofilaxia.
CONCLUSÕES: O protocolo para prevenção de tromboembolismo venoso no Instituto Ivo Pitanguy foi eficaz, sem ocorrência de eventos tromboembólicos e com incidência de hematomas abaixo da encontrada na literatura médica.
Palavras-chave: Tromboembolia venosa/prevenção & controle. Trombose venosa/prevenção & controle. Cirurgia plástica.
Thromboembolic events are a serious concern due to the high rates of morbidity and mortality as well as the possibility of existing disease presenting with scarce and often nonspecific symptoms.
Pulmonary embolism (PE) is the leading cause of death in hospitalized patients. Annually, 200,000 new cases occur; most of these cases have sudden onset and lead to death within the first 2 hours, even before therapy is initiated or has an effect1. Therefore, prevention is more effective than the treatment of the established disease.
Surgery is an important factor in the genesis of thromboembolism, because surgery is associated with several predisposing factors such as tissue trauma, decubitus, restricted movement, hypovolemia, and blood stasis. In the setting of plastic surgery, it is particularly important to highlight the fact that most cosmetic surgeries are performed on women in age groups wherein contraceptives and hormone replacement therapy are used frequently.
The absolute risk of deep vein thrombosis (DVT) in hospital settings is estimated to be 15-40% in surgical patients; a study published in 2009 reports that 80% and 53% of plastic surgeons have experienced DVT and PE, respectively2.
In 2001, Reinisch et al.3 reported that patients undergoing surgery of the face have DVT and pulmonary thromboembolism rates of 0.35% and 0.14%, respectively. In 2003, Aly et al.4 reported the incidences of pulmonary thromboembolism in patients undergoing circumferential abdominoplasty and abdominoplasty associated with another surgery to be 9.3% and 6.6%, respectively.
The risk stratification for thromboembolism, which is very common in clinical trials, is the basis for medical algorithms and protocols. It is also essential for streamlining costs as well as minimizing complications and adverse effects.
In 1994, Weinmann & Salzman5 scored the identified risk factors for thromboembolism and created a classification system for selecting the preventive procedure by dividing patients into low-, moderate-, and high-risk groups.
In 1999, a task force of the American Society of Plastic and Reconstructive Surgeons suggested some prophylactic measures for venous thromboembolism (VTE) but did not create a protocol6.
In 2001, the American College of Chest Physicians (ACCP) set some guidelines for the prevention of VTE in surgical patients, including knee flexion at approximately 5º to maximize blood flow in the popliteal veins, intermittent pneumatic compression in moderate-risk patients, administration of low-molecular-weight heparin (LMWH) in high-risk patients, and the maintenance of prophylaxis until the patient is walking normally.
A Brazilian report describing a protocol for preventing DVT in plastic surgery developed at the Israeli Albert Einstein Hospital, SP, was published in 2003 as a result of a multidisciplinary study that was initiated in 19997; this protocol uses the risk stratification previously used by Weinmann & Salzman5 in 1994.
Rohrich & Rios8 and Davison et al.9 claim that such care should be universal and point out that some cases deserve more aggressive measures, including patients undergoing abdominoplasty, combined surgeries, or procedures lasting more than 4 hours. Furthermore, Rohrich & Rios8 advocate early walking on the day of surgery and the routine preoperative use of LMWH.
In 2007, our institution established a prevention protocol10 based on the works of Davison et al.9 and Caprini et al.11. In 2009, in light of the updated ACCP guidelines (2008) and publication of new specific studies in plastic surgery, this protocol was modified in order ensure greater security and advocate earlier initiation of chemoprophylaxis.
However, surgeons are strongly resistant to adopting chemoprophylaxis due to the fear of increased bleeding and its complications.
This paper demonstrates the effectiveness of the aforementioned protocol for the prevention of thromboembolic diseases established at the Ivo Pitanguy Institute and demonstrates the equivalence in the rates of bruises with and without the use of LMWH.
METHODS
A retrospective study was performed between May 2009 and May 2010 involving patients undergoing plastic surgery (both cosmetic and reconstructive) at the Ivo Pitanguy Institute. All patients signed an informed consent form and were informed about the current protocol for the prevention of VTE.
All patients were assessed for risk factors, including predisposing or exposure factors for VTE. They were classified according to the degree of risk and subsequently received the recommended prophylaxis.
The prevention protocol of the service applied from 2007-2009 was based on the recommendations of the Davison-Caprini American protocol, which classifies patients into 4 risk categories: low risk (sum of risk factors, 0-1), moderate risk (2), high risk (3-4), and very high risk (> 4). According to this protocol, LMWH chemoprophylaxis is indicated only for the very high-risk category and always only 12 hours after surgery12,13.
The current protocol for the prevention of thromboembolic disease is based on the ACCP guidelines with certain adaptations by combining the models developed by Patronella et al.12, Young & Watson13, and Anger et al.7 (Appendix 1). As noted in Appendix 1, patients are assessed for the risk factors to which they are exposed as well as predisposing factors, thereby generating a score that was used to select the prophylaxis to be adopted.
All patients are prompted to walk on the day of surgery. The use of elastic stockings, starting after the procedure for 1 week, is suitable for all patients. Pneumatic compression is initiated after anesthetic induction and maintained until the next day.
In the adapted ACCP protocol, patients are stratified into 4 risk categories: low (total of up to 2 points), moderate (3-4 points), high (5-6 points), and very high risk (> 7). Drug prophylaxis is already administered in patients classified as moderate risk, starting 12 hours after the procedure, and the duration between the recommended drug prophylaxis administration and the surgery decreases according to the risk: 6 hours after for high-risk classification and 1 hour before the procedure for very high-risk classification). Patients with high and very high risks should be preoperatively evaluated by Doppler ultrasound of the lower limbs and maintained on LMWH for 2-5 days postoperatively. In cases of large displacements, we evaluate the use of half the dose of LMWH. Moreover, in patients undergoing spinal locks, LMWH is administered only after 12 hours.
The protocol can be modified by reducing or even discontinuing LMWH administration based on the medical criteria and the surgical complications that may develop.
Since the introduction of the new protocol for the prevention of VTE, all adverse events have been meticulously recorded, including the time of hematoma formation and completion of chemoprophylaxis, thus avoiding biases.
In 2010, the Subcommittee on the Control of Anticoagulation of the International Society on Thrombosis and Hemostasis (SITH) defined hematoma as any bleeding at the surgical site requiring further surgical intervention.
RESULTS
We evaluated a total of 1351 patients; 9.8% and 90.2% were men and women, respectively. The mean age was 40.8 years, ranging from 2-86 years.
Of the surgeries performed, 1246 (92.2%) were single and 105 (7.8%) were combined procedures. With regard to the type of surgery, mammoplasty was most common, with 19.7% of the procedures, followed by breast implants (16.9%), abdominoplasty (16.7%), rhytidectomy (13.6%), rhinoplasty (7.5%), liposuction (6.4%), blepharoplasty (5%), and other procedures (14.2%, including otoplasties, microimplant capillary, excision of skin lesions, buttock implants, calf implants, and palate reconstruction) (Figure 1).

Figure 1 - Percentages of specific surgeries performed.
According to the protocol, 34.6%, 58%, 6.7%, and 0.7% of patients were classified as low, moderate, high, and very high risk, respectively (Figure 2). The moderate risk group was the largest, and patients had already received chemoprophylaxis.

Figure 2 - Distribution of patients according to the risk group.
There were no cases of VTE during the study period. There were 16 (1.18%) cases of hematoma, including 9 (56.25%) after prophylaxis with heparin and 7 (43.75%) without the use of chemoprophylaxis (Figure 3). We identified 8 hematomas after facelift, 2 after liposuction, 3 after breast implant, 1 after mammoplasty, 1 after abdominoplasty, and 1 after breast expander placement. With regard to the distribution of hematomas according to type of prophylaxis performed, 43.75% of hematomas occurred in patients who did not use heparin, 43.75% of hematomas occurred in patients receiving heparin administration 12 hours after surgery, and 12.5% of hematomas occurred in patients receiving heparin administration 6 hours after surgery; there were no hematomas in patients who used heparin 1 hour before surgery (Figure 4). Of the 16 patients who had hematomas, 5 were hypertensive and 3 were dyslipidemic and were taking statins.
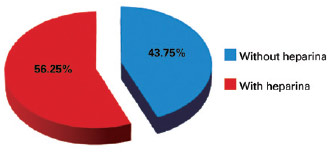
Figure 3 - Percentages of hematomas occurring with and without chemoprophylaxis.
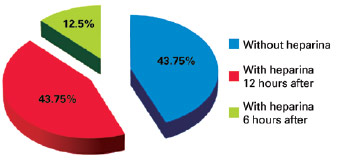
Figure 4 - Percentages of hematomas according to the prophylaxis used.
DISCUSSION
In our service, all patients undergoing surgical procedures are rigorously clinically evaluated. This evaluation considers the surgery to be performed, type of anesthesia, and patient's degree of risk. After the appropriate prophylaxis is selected, intraoperative monitoring is performed and postoperative surveillance is continued.
The prevention protocol is individualized; it is the basis for the postoperative clinical conduct and may be modified according to exceptional conditions encountered during surgery, taking into account the preoperatively predicted risk and the risk/benefit of pre-determined conduct.
In the first consultation, the patient is instructed to discontinue any medications with thrombogenic potential, such as oral contraceptives and hormone replacement therapy, 1 month before and up to 2 weeks after the surgery, when they are expected to be able to walk normally. Several preoperative instructions are given to patients who will be travelling in the days before the surgery. Some points are reinforced, such as not drinking alcohol 48 hours before surgery, drinking liquids during their trip, frequently moving their legs and walking every 2 hours when possible, and wearing elastic stockings for moderate compression if there are no contraindications.
Upon discharge, the guidelines include high liquid intake, frequent walking, and using elastic stockings for 1 week.
According to the recommendations in the 2008 ACCP guidelines as well as published studies, chemoprophylaxis should begin before or immediately after surgery and continue until the patient walks normally. In the present study, heparin administered 2 hours before or 4-6 hours after surgery, at half the usual dose, reduced the formation of thrombus compared to treatment initiated 12 hours or more after surgery. Furthermore, the risk of hemorrhagic events was similar in both groups. Patronella et al.12 conducted a retrospective study on 3871 patients and recommended administering folic acid and complex B vitamins 2 weeks before surgery, preventing hypothermia, altering bed position to achieve 5º flexion of the knees, using stockings and external pneumatic compression of the lower limbs, administering 40 mg LMWH 1 hour after surgery in high-risk patients once a day for 72 hours, and monitoring the patient for 4 weeks.
A study published by Newall et al.14 demonstrates that LMWH administered 1 hour after surgery and continued for 3 consecutive days in high-risk patients results in the absence of VTE without increasing hematoma rates compared to those in the literature.
In our protocol, heparin treatment starts 1 hour before surgery in the high-risk group, aiming to reach the peak of action during the actual surgical procedure.
Despite the risk of VTE, there is still much reluctance to administer chemoprophylaxis due to the risk of bleeding complications. In addition, the appropriateness of the development of these guidelines is debatable due to the limited data on plastic surgery available in the literature. Concerns include the actual incidence of VTE in plastic surgery, frequencies of complications, optimal time to initiate prophylaxis, and optimal dose. It should be noted that the ACCP guidelines do not include plastic surgery and there are no published studies with evidence level A or B for the establishment of protocols in plastic surgery. However, the need for prevention protocols is imperative, because VTE is the most important preventable postoperative cause of death.
A rational preventive strategy encompassing the risk conditions of each patient should be established, and it could play a key role in the preoperative evaluation. The notion that the risk of VTE in plastic surgery patients is low is misleading, given that approximately two-thirds of patients with VTE are asymptomatic. The lack of attention to this issue is due to the lack of recognition of a problem that can have catastrophic consequences, because fatal pulmonary thromboembolism is often the first and only manifestation of the disease.
Delaying the initiation of prophylaxis leads to a suboptimal antithrombotic effect and does not confer any security advantage.
There is no evidence that heparin use in plastic surgery increases the risk of hematomas, as demonstrated in the report of Rohrich & Rios8.
Meta-analyses and double-blinded randomized clinical trials indicate little or no increase in the rate of bleeding with LMWH in surgeries2, 15.
Another study in plastic surgery performed by Liao et al.16 in 2008 shows that there was no increase in the risk of hematoma associated with heparin chemoprophylaxis after breast reconstruction using transverse rectus abdominis myocutaneous flaps.
The use of anticoagulants in patients undergoing facelift is generally avoided due to the risk of increased bleeding, which can cause massive hematomas as well as potential tissue necrosis.
Non-maleficence is one of the principles of medicine. Patients undergoing plastic surgery are generally healthy, have high expectations, and have a low tolerance for adversity. Therefore, the plastic surgeon should protect the safety of patients and prevent complications.
A review of 126 cases of rhytidectomy by the same surgeon indicates a 5.6% incidence of hematoma requiring surgical procedure and a 16.2% incidence of postoperative bleeding with the use of prophylactic LMWH administered 2 hours prior to surgery17. In the present study, the average operative time was 95 minutes, ranging from 45-145 minutes.
Pitanguy & Ceravolo18 studied hematomas in rhytidec-tomy and report that the rates of hematoma in the literature range from 0.9-8% without the use of prophylactic heparin.
In the present study, there were no cases of VTE and 16 cases of hematoma; of these, 9 were observed after chemoprophylaxis and 7 were observed without the use of chemoprophylaxis. The largest group of patients in the present study had received heparin 12 hours after surgery. Hematomas were not observed in patients who received heparin 1 hour before surgery. These findings support the idea that the risk factors for bleeding are more important than the role of heparin in the development of hematoma.
Of the 9 patients who had hematoma following heparin use, 3 were hypertensive and 3 were dyslipidemic-1 of these patients regularly took statins, which have an anticoagulant effect. The shortest time interval for the appearance of hematoma was 5 hours after heparin administration, which coincides with the peak action time of the drug, whereas the longest interval was 16 hours.
It is important to note that 43.8% of hematoma cases did not involve anticoagulant medication.
The incidence rates of hematoma in plastic surgery published in the literature, which are the rates we use as references, are as follows: 1.1-2.0% in breast augmentation with prosthesis19, 4.5-8% in rhytidectomy, 3-4.6% in abdominoplasty20, 2-10% in lipoplasty, and 0.7% in reduction mammoplasty. With regard to liposuction, there is no reported difference between seroma and hematoma, thus making it difficult to define a specific incidence of hematoma.
The incidence rates of hematoma observed in the present study are lower than those reported in the literature. It should be noted that we are comparing statistical values derived from different clinical situations. Therefore, this further corroborates the fact that the use of LMWH does not increase the incidence of hematoma.
CONCLUSIONS
The protocol for the prevention of VTE at the Ivo Pitanguy Institute is effective, and no thromboembolic events have been recorded. The incidence of hematomas is below that reported in the medical literature.
The Ivo Pitanguy Institute has always demonstrated excellence and is a pioneer in education, research, and healthcare practice in aesthetic and reconstructive plastic surgery. It is necessary to provide patients the best surgical outcome; this always involves providing the highest level of safety, which often involves changing habits and behaviors. Meticulous prevention based on well-established criteria is the best way to avoid deaths as well as the high cost of examinations and complementary treatments.
REFERENCES
1. King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs. three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis. Chest. 2007;131(2):507-16.
2. Miszkiewicz K, Perreault I, Landes G, Harris PG, Sampalis JS, Dio-nyssopoulos A, et al. Venous thromboembolism in plastic surgery: incidence, current practice and recommendations. J Plast Reconstr Aesthet Surg. 2009;62(5):580-8.
3. Reinisch JF, Bresnick SD, Walker JW, Rosso RF. Deep venous thrombosis and pulmonary embolus after face lift: a study of incidence and prophylaxis. Plast Reconstr Surg. 2001;107(6):1570-5.
4. Aly AS, Cram AE, Chao M, Pang J, McKeon M. Belt lipectomy for circumferential truncal excess: the University of Iowa experience. Plast Reconstr Surg. 2003;111(1):398-413.
5. Weinmann EE, Salzman EW. Deep-vein thrombosis. N Engl J Med. 1994;331(24):1630-41.
6. McDevitt NB. Deep vein thrombosis prophylaxis. American Society of Plastic and Reconstructive Surgeons. Plast Reconstr Surg. 1999;104(6):1923-8.
7. Anger J, Baruzzi ACA, Knobel E. Um protocolo de prevenção de trombose venosa profunda em cirurgia plástica. Rev Bras Cir Plást. 2003;18(1):47-54.
8. Rohrich RJ, Rios JL. Venous thromboembolism in cosmetic plastic surgery: maximizing patient safety. Plast Reconstr Surg. 2003;112(3):871-2.
9. Davison SP, Venturi ML, Attinger CE, Baker SB, Spear SL. Prevention of venous thromboembolism in the plastic surgery patient. Plast Re-constr Surg. 2004;114(3):43e-51e.
10. Paiva RA, Pitanguy I, Amorim NFG, Berger R, Shdick HA, Holanda TA. Tromboembolismo venoso em Cirurgia Plástica: protocolo de prevenção na Clínica Ivo Pitanguy. Rev Bras Cir Plást. 2010;25(4):583-8.
11. Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol. 2001;38(2 Suppl 5):12-9.
12. Patronella CK, Ruiz-Razura A, Newall G, Mentz HA, Arango ML, As-savapokee T, et al. Thromboembolism in high-risk aesthetic surgery: experience with 17 patients in a review of 3871 consecutive cases. Aesthet Surg J. 2008;28(6):648-55.
13. Young V, Watson M. The need for venous thromboembolism (VTE) prophylaxis in plastic surgery. Aesthet Surg J. 2006;26(2):157-75.
14. Newall G, Ruiz-Razura A, Mentz HA, Patronella CK, Ibarra FR, Zarak A. A retrospective study on the use of a low-molecular-weight he-parin for thromboembolism prophylaxis in large-volume liposuction and body contouring procedures. Aesthetic Plast Surg. 2006;30(1):86-95.
15. Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):287S-310S.
16. Liao EC, Taghinia AH, Nguyen LP, Yueh JH, May JW Jr, Orgill DP. Incidence of hematoma complication with heparin venous thrombosis prophylaxis after TRAM flap breast reconstruction. Plast Reconstr Surg. 2008;121(4):1101-7.
17. Durnig P, Jungwirth W. Low-molecular-weight heparin and postoperative bleeding in rhytidectomy. Plast Reconstr Surg. 2006;118(2):502-9.
18. Pitanguy I, Ceravolo MP. Hematoma postrhytidectomy: how we treat it. Plast Reconstr Surg. 1981;67(4):526-9.
19. Codner MA, Mejia JD, Locke MB, Mahoney A, Thiels C, Nahai FR, et al. A 15-year experience with primary breast augmentation. Plast Reconstr Surg. 2011;127(3):1300-10.
20. Stewart KJ, Stewart DA, Coghlan B, Harrison DH, Jones BM, Wa-terhouse N. Complications of 278 consecutive abdominoplasties. J Plast Reconstr Aesthet Surg. 2006;59(11):1152-5.
1. Master of Intensive Care Medicine, Federal University of Rio de Janeiro (UFRJ), specialist in intensive care medicine, head of the intensive care unit at the Ivo Pitanguy Institute, Rio de Janeiro, RJ, Brazil
2. Cardiologist, trained at the National Institute of Cardiology of Rio de Janeiro, member of the clinical staff of the Intensive Care Unit at the Ivo Pitanguy Institute, Rio de Janeiro, RJ, Brazil
3. Full member of Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery - SBCP), assistant professor in the department of plastic surgery at the Pontifical Catholic University of Rio de Janeiro (PUC-Rio), Rio de Janeiro, RJ, Brazil
4. Associate member of the SBCP, assistant professor in the department of plastic surgery at PUC-Rio, Rio de Janeiro, RJ, Brazil
5. Patron of the SBCP, member of the National Academy of Medicine and the Brazilian Academy of Letters, Professor of Post-Graduation in Plastic Surgery at PUC-Rio and Institute Carlos Chagas, Rio de Janeiro, RJ, Brazil
Correspondence to:
Rita Azevedo de Paiva
Rua Visconde de Pirajá, 330 - ap. 604 - Ipanema
Rio de Janeiro, RJ, Brazil - CEP: 22410-001
E-mail: ritaazevedodepaiva@hotmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: August 14, 2012
Article accepted: January 13, 2013
This study was performed at the Ivo Pitanguy Institute, Rio de Janeiro, RJ, Brazil.



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter