

Original Article - Year 2012 - Volume 27 -
Experimental study of adequate microwave warming of crystalloids and derivation of an equation for calculating heating parameters
Estudo experimental do aquecimento adequado de solução cristaloide por micro-ondas e dedução de equação para seu cálculo
ABSTRACT
BACKGROUND: Crystalloids are commonly heated in microwave ovens for intravenous or subcutaneous usage (e.g., in liposuction). However, few studies have empirically determined the parameters for this procedure. This study experimentally evaluated the heating of saline solution (sodium chloride 0.9%) at different initial temperatures to derive an equation to calculate heating parameters as well as the temperature decrease in saline solutions after heating.
METHODS: The initial temperature of intravenous pouches containing saline solution (500 and 1,000 mL) was adjusted to 15ºC, 20ºC, or 25ºC. The 500 and 1,000 mL pouches were then warmed in a microwave (900 W) for 60 and 120 seconds, respectively. The temperature of the saline solution was measured during heating and immediately and 30 minutes after heating. An equation was derived from the results.
RESULTS: Warming the 500 and 1,000 mL pouches for 60 and 120 seconds, respectively, at 900 W increased the temperature of these pouches by 20ºC. The derived equation was as follows: final temperature = initial temperature + [0.165 × time (s)/volume (L)]. No significant differences were found between the internal and external temperatures of the pouches after heating.
CONCLUSIONS: Determining the initial temperature of crystalloid solutions is essential for obtaining the desired temperature after microwave warming. The equation derived in the present study enables calculation of the final temperature of solutions with various initial temperatures. The external temperature of the pouches accurately reflects their internal temperature.
Keywords: Hypothermia. Microwaves. Parenteralinfusions.
RESUMO
INTRODUÇÃO: Fluidos cristaloides são comumente aquecidos em forno de micro-ondas, para uso endovenoso ou subcutâneo (em lipoaspirações). A literatura médica é pobre em trabalhos estabelecendo parâmetros científicos para esse processo. O objetivo deste estudo foi estudar experimentalmente o aquecimento de solução salina (cloreto de sódio a 0,9%), a partir de diferentes temperaturas iniciais, deduzir uma equação para permitir o cálculo de parâmetros de aquecimento e estudar o decréscimo de temperatura da solução aquecida.
MÉTODO: Frascos para infusão endovenosa (500 ml e 1.000 ml) de solução salina tiveram sua temperatura inicial ajustada para 15ºC, 20ºC e 25ºC, sendo aquecidos por micro-ondas até 60 segundos (500 ml) e 120 segundos (1.000 ml). A temperatura da solução salina foi medida durante e ao final do aquecimento e até 30 minutos após esse processo. A partir dos resultados, foi deduzida uma equação.
RESULTADOS: O aquecimento por 60 segundos elevou a temperatura de frascos de 500 ml de solução salina em cerca de 20ºC. Foram necessários 120 segundos para a mesma elevação de temperatura de frascos de 1.000 ml. A equação deduzida foi: TIF = TII + [0,165 x Tempo (segundos) / Volume (litros)], onde TIF = temperatura interna final e TII = temperatura interna inicial. Não foram encontradas diferenças significantes entre as temperaturas interna e externa após o aquecimento.
CONCLUSÕES: A determinação da temperatura inicial é fundamental na obtenção da temperatura final desejada, após o aquecimento de fluidos cristaloides por micro-ondas. Uma equação pôde ser deduzida, tornando possível o cálculo da temperatura final a partir de várias temperaturas iniciais. A temperatura externa dos frascos reflete adequadamente sua temperatura interna.
Palavras-chave: Hipotermia. Micro-ondas. Infusões parenterais.
Hypothermia is characterized by a body temperature of < 35ºC. It often occurs in emergency rooms, especially in cold regions, where patients are overly exposed or kept in wet clothing. Data from the Centers for Disease Control and Prevention show that in very young or very old patients or patients with problems such as hypothyroidism, infections, and alcoholism, hypothermia can cause mortality rates between 65% and 90%1.
Anesthesia, either general or local, contributes to heat loss by limiting muscle activity, reducing metabolism, and respiratory work2. Besides anesthesia, surgical patients may be prone to hypothermia through mechanisms such as preoperative bathing, insufficient clothing, inadequate transportation of the patient to the hospital, low operating room temperature, application of cold antiseptic solutions, and use of cold crystalloid solutions, either to irrigate cavities or for intravenous usage3.
During liposuction, subcutaneous infusion of large volumes of cold solutions can be an important factor contributing to hypothermia. When this occurs during post-anesthetic recovery, hypothermia can induce significant shivering; this can increase oxygen requirements by up to 500%, thus generating serious complications such as arrhythmias and myocardial infarction in patients with coronary artery disease4. The maintenance of intraoperative normothermia reduces the incidence of morbid cardiac events5.
Warming subcutaneous and intravenous solutions may be essential to prevent postoperative hypothermia, because the decrease in body temperature induced by cold liquids may be significant when large volumes of parenteral solutions are administered6.
The use of microwave ovens to warm intravenous crystalloid solutions such as sodium chloride 0.9% (i.e., saline solution) began in 19807. This is a simple, safe, fast, widely available, and cost-effective method8. Although widely used, only one article published in Thailand9 reports precise parameters for this warming procedure.
The initial temperature has not been taken into consideration in the warming of solutions. The usual procedure is to place a 500 mL pouch in a microwave oven for 60 seconds at full power; when 1,000 mL pouches are used, the time is usually set to 120 seconds. Because the final temperature depends on the initial temperature, considerable differences may exist if imprecise parameters are used. This can lead to either insufficient or excessive heating of solutions. If a solution is too warm, it may cause burns and venous thrombosis10.
In plastic surgery, operative hypothermia is reported to be present in 20% of body-contouring procedures after bariatric surgery and is associated with increased risks of seroma, blood loss, and transfusion requirements11.
During ambulatory aesthetic surgery, measures to prevent hypothermia can reduce the need for intraoperative analgesia and are associated with a shorter time in the recovery room and earlier discharge12.
In this study, experiments were performed to establish correlations between the initial and final temperatures of a crystalloid solution (saline) heated in a microwave oven under controlled conditions in order to derive an equation for calculating the heating parameters.
The decrease in temperature of the heated solution and the relationship between the internal and external temperatures of saline solution pouches were also studied.
METHODS
All experiments were performed in a temperature-controlled operating room (24ºC-25ºC). Thirty-six pouches of crystalloid fluid (0.9% sodium chloride [saline], 500 or 1,000 mL; JP Laboratory Pharmaceutical Industries SA, Ribeirão Preto, Brazil) were divided into 6 groups containing 6 bags each initially heated to different temperatures:
Group 1 - 500 mL at 15ºC; Group 2 - 500 mL at 20ºC; Group 3 - 500 mL at 25ºC; Group 4 - 1,000 mL at 15ºC; Group 5 - 1,000 mL at 20ºC; Group 6 - 1,000 mL at 25ºC.
The external temperature of all bags was measured using a laser thermometer (Infrared TD960) positioned 20 cm away from each pouch. The internal temperature of the pouch (i.e., the solution) was measured using a digital thermometer (Sigma Digi-Thermo) the sensor being introduced through the injection port. A microwave oven set at a maximum power of 900 W and a frequency of 2,450 MHz (Consul M.V31AO, São Paulo, Brazil) was used to warm the pouches.
The 500 mL pouches were heated for 30, 45, or 60 seconds. The 1,000 mL pouches were heated for 30, 45, 60, or 120 seconds.
Upon warming, each pouch was stirred to homogenize its contents, placed in a plastic holder, and fixed to an IV holder. The opening of the pouch was positioned upward to facilitate the introduction of the digital thermometer sensor. The temperature was measured immediately after warming; measurements were repeated 4 times at 10 minute intervals to measure the temperature decrease.
The data were tabulated and statistically analyzed using SAEG90 and Minitab 14. Regression analysis was performed. The level of significance was set at P < 0.05.
RESULTS
After a 60 second warming at 900 W, the mean internal temperature of the 500 mL saline solution pouches increased by 20.3ºC. After a 120 second warming, the mean internal temperature of the 1,000 mL saline solution pouches increased by 19.1ºC (Tables 1 and 2).


The following equation was derived from the temperature readings:
TIF = TII + [0.165 × time (s)/volume (L)] ± 0.6ºC
where TIF is the final internal temperature, TII is the initial internal temperature, and 0.165 is the average constant obtained from the data.
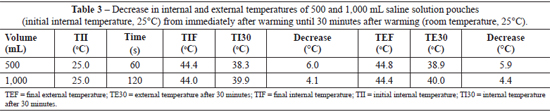
After 30 minutes, the mean internal temperature decreased by 5.1ºC (Table 3).
No significant differences were found between the internal and external temperatures.
DISCUSSION
The importance of warming fluids for intravenous, subcutaneous and intraperitoneal use has been long established and seems beyond discussion. This is significantly beneficial to patients, achieving more stable hemodynamic variables and higher core temperature at the end of operations13. Yet, the means to attain such warming should be better studied. A common practice is the storing of parenteral fluids in deposits where the temperature isn't controlled. These fluids' temperatures tend to equal that of the room where they lay. This may lead to significant temperature differences between stored solutions at summer or wintertime. Heating such solutions by microwaves, using always the same parameters, can lead to significantly different end temperatures, if the initial temperatures are also quite diverse. The usual procedure is to set microwave ovens, whatever their model and power output, to maximal power and warm 500 mL saline pouches during one minute, doubling this time for 1,000 mL pouches, irrespectively of the initial temperature. As it has been verified in this study - should be predicted - the end temperature indeed depends on the initial one.
An hypothetical example could be considered: in wintertime, at a southern state in Brazil, a victim of significant burns is treated at a hospital devoid of environmental heating. In this place, room temperature is 15ºC, and so is that of stored saline solutions. The patient has hypovolemic shock and demands high-volume crystalloid infusion. 1,000-mL saline pouches are heated by microwaves (considering a 900 W device) during 2 min. The end temperature of these saline pouches will be 34ºC, as shown in this work. After an important amount of fluid administration, the patient develops signs of hypothermia, which worsens the prognosis. No wonder - the fluid temperature was quite lower than would be adequate.
Another example, contrary to the aforementioned one: in summertime, at a northern state, also in Brazil, a similar patient is treated at a hospital devoid of air-conditioning. In this place, room temperature is 30ºC, and so is that of stored saline solutions. The patient demands high-volume reposition. 1,000 mL saline pouches are heated by microwaves (also considering a 900 W oven) during 2 min. The end temperature of these saline pouches, calculated using the equation deducted by us, will be no less than 49ºC - a very dangerous one. Such examples are eloquent in expressing the importance of initial temperature and of adequate warming parameters. In this sense, being aware of such variations, using the results presented in this work, and being always aware of the initial temperature of fluids, can make a significant difference in the treatment of patients who need high-volume crystalloid reposition.
The rate of temperature decrease, after microwave heating, was relatively slow in this work, when room temperature was 25ºC (a decrease of 5.9ºC for 500 mL pouches and of 4.4ºC for 1,000 mL pouches, after 30 min). It can be assumed, then, that such rate isn't an important consideration, when heated fluids are administered with speed, as it mostly happens in cases with significant hypovolemia. When infusion is slow, however, the fluid's temperature can fall to inadequate levels, something that should be considered in the prevention of hypothermia.
It is very important that environmental thermometers are available in hospitals, in order to measure room temperature. Provided that saline pouches have been stored, at the rooms whose temperature is quantified through such thermometers, for a time long enough to make pouch temperature approach that of the environment, more exact measurements could be unnecessary. More precise (and expensive) devices, like laser thermometers, to estimate external pouch temperature, would be welcome, but they aren't essential when this work's data are used. The most important results can be summarized as follows: warming 500 ml pouches of saline in a 900 W microwave oven during 60 s will produce a temperature increase of about 20ºC, whereas 1,000 ml pouches need 120 s to gain approximately the same. To obviate the need for calculations, tables can be constructed, containing different initial temperatures and the time (in seconds) necessary to raise fluid temperature up to 38-40ºC in a 900 W microwave oven set to maximal power. Such tables should be always available - and used - at emergency rooms, wards and operating facilities.
It is important to state that our findings apply to 900 W microwave ovens. More potent devices should have their power set to 900 W, in order to reproduce the conditions of this work. Using devices with less than 900 W maximum output can demand longer times; this has not been studied in this work. In Brazil, microwave ovens are sold by great magazines with maximum power outputs varying from 700 to 1,500 W. Using ovens with more than 900 W at full power during 60 s (500 ml) or 120 s (1,000 ml) will overheat the solution, except when its initial temperature is too low14.
Automated intravenous fluid-heating machines can be used, obviating the need for microwave ovens and making calculations, but their price makes them a luxury item at so many hospitals and outpatient facilities, especially in developing countries. In such places, the findings of this work can be useful.
The plastic surgeon should always try to prevent hypothermia, which may appear even in relatively short operations. Simple measures, such as warming the patient pre-operatively, keeping an adequate operating room temperature, avoiding wide exposure of the patient's body surface, preventing post-operative shivering, and, of course, warming of intravenous and subcutaneous fluids, can be very important in reducing complications and adverse outcomes15. In this sense, the rational use of microwave ovens, instead of blindly following the same pattern of heating irrespective of circumstances, can be a very important component of highquality surgical care.
CONCLUSIONS
Determining the initial temperature of saline solution is critical for maintaining a desired final temperature after warming in a microwave oven. The equation derived in the present study makes it possible to calculate the final temperature from different initial temperatures when using a microwave oven at 900 W. The external temperature accurately reflects the internal temperature of saline solution pouches.
REFERENCES
1. Centers for Disease Control and Prevention. Hypothermia-related deaths: Suffolk County, New York, January 1999-March 2000, and United States, 1979-1998. MMWR Morb Mortal Wkly Rep. 2001;50(4):53-7.
2. Young CC, Sladen RN. Temperature monitoring. Int Anesthesiol Clin. 1996;34(3):149-74.
3. Pisani IS. Prevenção da hipotermia per-operatória e a utilidade do forno de micro-ondas. Rev Bras Anestesiol. 1999;49(6):399-402.
4. Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC. Manual de anestesiologia clínica. 6ª ed. Porto Alegre: Artmed; 2010.
5. Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: a randomized clinical trial. JAMA. 1997;277(14):1127-34.
6. Esnaola NF, Cole DJ. Perioperative normothermia during major surgery: is it important? Adv Surg. 2011;45:249-63.
7. Bagatini A, Nascimento L. Aquecimento de soluções cristalóides em forno de microondas: segurança e toxicidade. Rev Bras Anestesiol. 1997;47(3):237-44.
8. Werwath DL, Schwab CW, Scholten JR, Robinett W. Microwave ovens: a safe new method of warming crystalloids. Am Surg. 1984;50(12):656-9.
9. Chittawatanarat K, Akanitthaphichat S. Microwave oven: how to use it as a crystalloid fluid warmer. J Med Assoc Thai. 2009;92(11):1428-33.
10. Sieunarine K, White GH. Full-thickness burn and venous thrombosis following intravenous infusion of microwave-heated crystalloid fluids. Burns. 1996;22(7):568-9.
11. Coon D, Michaels J 5th, Gusenoff JA, Chong T, Purnell C, Rubin JP. Hypothermia and complications in postbariatric body contouring. Plast Reconstr Surg. 2012;130(2):443-8.
12. Lista F, Doherty CD, Backstein RM, Ahmad J. The impact of perioperative warming in an outpatient aesthetic surgery setting. Aesthet Surg J. 2012;32(5):613-20.
13. Moola S, Lockwood C. Effectiveness of strategies for the management and/or prevention of hypothermia within the adult perioperative environment. Int J Evid Based Healthc. 2011;9(4):337-45.
14. Delaney A. Reliability of modern microwave ovens to safely heat intravenous fluids for resuscitation. Emerg Med (Fremantle). 2001;13(2):181-5.
15. Young VL, Watson ME. Prevention of perioperative hypothermia in plastic surgery. Aesthet Surg J. 2006;26(5):551-71.
1. Plastic Surgeon, PhD, full member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery - SBCP), Professor at the Universidade Vale do Rio Verde (University Vale do Rio Verde - UNINCOR), Três Corações, MG, Brazil.
2. Nurse, master, Coordinator of the Nursing Course in UNINCOR, Três Corações, MG, Brazil.
3. Zootechnician, doctor and master in Food Sciences, Professor of UNINCOR, Três Corações, MG, Brazil.
Correspondence to:
Tufi Neder Meyer
Rua Edson Arantes do Nascimento, 201 - Centro
Três Corações, MG, Brazil - CEP 37410-000
E-mail: tufi@uai.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: August 26, 2012
Article accepted: October 15, 2012
This study was performed at the Universidade Vale do Rio Verde(University Vale do Rio Verde - UNINCOR), Três Corações, MG, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter