

Original Article - Year 2012 - Volume 27 -
Treatment of superficial second degree burn of face and neck with topical heparin: a comparative, prospective and randomized study
Tratamento de queimadura de segundo grau superficial em face e pescoço com heparina tópica: estudo comparativo, prospectivo e randomizado
ABSTRACT
BACKGROUND: New treatment options for thermal injuries are very desirable, especially if they reduce healing time and pain without increase of infection rates. Recent studies suggest that heparin topical use can achieve those goals. This study has the objective to evaluate healing time, pain and infection rate comparing topical use of heparin and collagenase in the treatment of superficial second degree burns of face and neck.
METHODS: Twenty patients were randomized into 2 groups: group treated with topical heparin and group treated with collagenase (control group). The exclusion criteria were: history of bleeding, blood discrasia, allergies to the product, active peptic ulcer and burns with more than 24 hours. Mann-Whitney test was applied to evaluate the results. The pain was measured by the use of opioid analgesics.
RESULTS: The heparin was not effective in decrease of healing time nor the use of opioids, and the infection rate didn't present significant difference between the groups.
CONCLUSIONS: The heparin can be used safely in treatment of superficial second degree burn of face and neck, but its beneficial effects need to be proven.
Keywords: Burns/therapy. Head. Neck. Heparin.
RESUMO
INTRODUÇÃO: Novas opções terapêuticas para o tratamento de lesões térmicas são constantemente buscadas, especialmente se reduzirem tempo de cicatrização e dor, sem aumentar as taxas de infecção das queimaduras. Estudos recentes sugerem que o uso tópico de heparina pode alcançar esses objetivos. Este estudo tem o objetivo de avaliar tempo de epitelização, dor e taxa de infecção, comparando o uso de heparina tópica ao uso de colagenase no tratamento de queimadura de segundo grau superficial de face e pescoço.
MÉTODO: No total, 20 pacientes foram randomizados em dois grupos: grupo tratado com heparina sódica e grupo tratado com colagenase (controle). Os critérios de exclusão foram: história de sangramento, discrasia sanguínea, alergias ao produto, úlcera péptica ativa e queimadura há mais de 24 horas. O teste de Mann-Whitney foi utilizado para avaliar os resultados. A dor foi avaliada pela necessidade do uso de analgésicos opioides.
RESULTADOS: A heparina não foi efetiva em diminuir o tempo de epitelização ou o uso de opioides, e a taxa de infecção não apresentou diferença estatística entre os grupos.
CONCLUSÕES: A heparina pode ser usada com segurança no tratamento de queimadura de segundo grau superficial em face e pescoço, mas seus efeitos benéficos ainda precisam ser comprovados.
Palavras-chave: Queimaduras/terapia. Cabeça. Pescoço. Heparina.
Two main events summarize the physiopathology of burns: edema and an increase in capillary hydrostatic pressure. Thermal injury induces a cascade of systemic inflammatory reactions by the exposure of the subendothelial collagen. This cascade results in tissue edema and hypovolemia. Activation of the calicrein system produces cytokinins that aggravate both the edema and hypovolemia. Cytokinin and collagen exposure activate the arachnoid acid-phospholipase system, liberating prostaglandins and, thus, elevating capillary permeability. Locally, substance P, serotonin, nitric oxide, bradykinin and leukotrienes play a role in increased local capillary permeability.
One of the first authors to study the effect of heparin in thermal injuries was McCleery et al.1. He verified a favorable alteration in animals with the use of parenterally administered heparin sodium. Saliba Jr.2-4, the current author who most studies this subject, also demonstrated favorable effects on humans.
Second degree burns cause great pain to the patient, because the nerve endings are exposed. Superficial partial thickness burns heal in, at most, two weeks. Blistering is very common.
Multiple studies1-6 suggest that treating burns with topical heparin is of beneficial effect in various ways such as: healing time shortened by several days, reduced burn edema, reduced pain, anti-inflammatory effect, limited cell destruction, and better scar quality, without change in rates of infection. However, there is no strong evidence of those effects because of the poor quality of those articles.
This study aims to evaluate epithelialization time, pain and infection rate, comparing the use of heparin and the use of topical collagenase (control group) in the treatment of superficial second-degree burn of face and neck.
METHODS
After institutional review board approval, 20 patients were evaluated during a period July, 2006 through August, 2007.
Patients with superficial second degree burns of face and neck of less than 24 hours were candidates for our study.
Patients with a history of bleeding, blood dyscrasia, burns of more than 24 hours, allergies to one of the products, or active peptic ulcer were excluded from our study.
After initial evaluation, the treatment was randomized by raffle of 20 envelopes (10 for each group) kept in a safe.
The patients were informed of the study and after this, signed a consent term of their participation, with possible complications, ethical and legal implications.
On the heparin group, we performed rupture of the burn blisters, cleaned the burn wound with chlorexidine and applied topical heparin (10.000 UI/ml) with 3 "puffs" of spray three times a day until the formation of the crusts (Figures 1 to 3). The heparin was then applied two times a day at a 20 cm distance totalizing 1% of the burn.
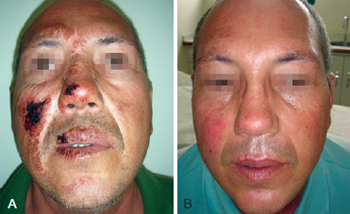
Figure 1 - In A, formation of crusts after heparin administration. In B, complete healing.
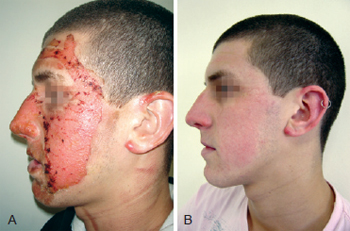
Figure 2 - In A, formation of crusts after heparin administration. In B, complete healing.
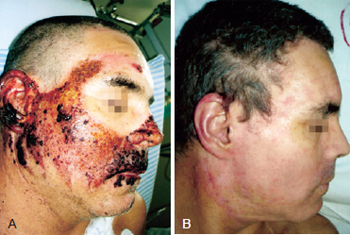
Figure 3 - In A, formation of crusts after heparin administration. In B, complete healing.
On the collagenase group, we performed rupture of the burn blisters, cleaned the burn wound with chlorexidine and applied a thin layer of collagenase one time a day (Figures 4 and 5).
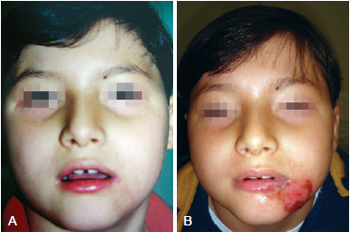
Figure 4 - In A, appearance after application of collagenase. In B, complete healing.
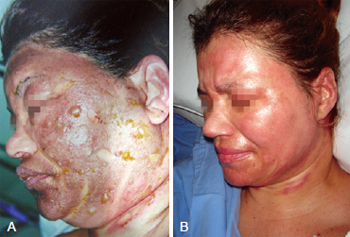
Figure 5 - In A, initial appearance of the burn before the application of collagenase. In B, complete healing.
Both groups were evaluated as to need for hospitalization, time elapsed between burn and treatment, total body surface area, need for analgesics (evaluation of pain), healing time, and rate of infection.
The Mann-Whitney test was used with a significance level of 5%.
RESULTS
A total of 20 patients with burns of face and neck were enrolled prospectively, ten treated with collagenase (control group) and 10 treated with topical heparin.
Average age was 22.85 years, ranging from 1 to 58 years. The main cause of burns was flames (40%). There was no bleeding or allergies to the product.
The percentage of total body surface area (TBSA) varied from 0.5% to 47%.
The time elapsed between burn and treatment was on average 11 hours and 1 minute (variation = 30 minutes to 24 hours). Seven patients required hospitalization.
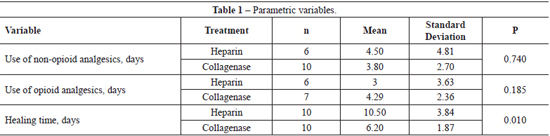
Time of epithelization varied from 5 to 17 days with healing, on average, in 10.5 days for the topical heparin group and 6.2 days for the collagenase group (P=0,010).
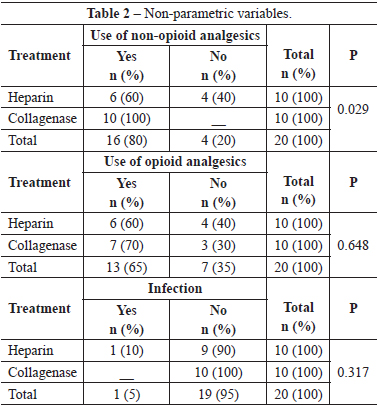
Patients of the heparin group needed less non-opioid medicine than those of the collagenase group (P=0.029). However, there was no significant difference between the use of opioids in both groups (P=0.648).
Only one of the patients enrolled had wound infection in the heparin group; nevertheless, there was no statistical relevance (P=0.317). One patient had hyperpigmentation and another, hypertrophic scarring.
The results of analysis of the parametric and non-parametric variables are presented respectively in Tables 1 and 2.
DISCUSSION
The treatment of burns with topical heparin is another choice in burn therapy. Heparin is a glycosaminoglycan, are highly acidic long chain compounds and with a negative charge. Heparin is the most sulfated and acidic2. The treatment of burns with heparin has been advocated by some authors because of its supposed anti-inflammatory and neoangiogenic effects3.
Superficial second-degree burns of face and neck have, in most cases, been treated, with open topical ointments. Open topical collagenase is the standard treatment for burns of the face and neck at our institution. Burns on the face and neck need special care because of possible damage to upper airway, ear cartilage and eye; likewise, of cicatricial microstomy and cervical contracture.
A number of non-comparative studies suggest that heparin reduces the healing time of burns leading to better quality scaring.
The results of our study comparing time of epithelization between both groups showed that the collagenase had a faster healing time as compared to the heparin group (P<0.05). This means that heparin does not exert a significant influence on healing time.
Results comparing pain showed us that the heparin group used less non-opioids analgesics than the collagenase group (P<0.05). This may have been so because the burn on the heparin group is not manipulated causing less pain or, because the heparin itself really exerts an analgesic effect. There was no significant difference between both groups in the use of opiods (P>0.05); hence, we can say that heparin was not effective in reducing use of opioids. This could have occurred due to the heterogeneity of the population studied with regard to the extent of surface burnt area, because the patients that used opioids had more extensive burnt areas.
The ideal topical agent should have the following characteristics: promote healing, control infection, be easy to apply and store, relieve pain and not soil the bed and clothing. Heparin does not have all of these characteristics.
CONCLUSIONS
Heparin can be applied safely in the treatment of second degree burns of face and neck, however its beneficial effects need to be proven.
REFERENCES
1. McCleery RS, Schaffarzick WR, Light R. An experimental study of the effect of heparin on the local pathology of burns. Surgery. 1949;26(3):548-64.
2. Saliba MJ Jr. Heparin in the treatment of burns: a review. Burns. 2001;27(4):349-58.
3. Saliba MJ Jr. The effects and uses of heparin in the care of burns that improves treatment and enhances the quality of life. Acta Chir Plast. 1997;39(1):13-6.
4. Saliba MJ Jr. Heparin efficacy in burns. II. Human thermal burn treatment with large doses of topical and parenteral heparin. Aerosp Med. 1970;41(11):1302-6.
5. Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al.; CONSORT GROUP (Consolidated Standards of Reporting Trials). The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663-94.
6. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Lancet. 2001;357(9263):1191-4.
1. Full member of the Sociedade Brasileira de Cirurgia Plástica/ Brazilian Society of Plastic Surgery (SBCP), associate physician of the Hospital dos Defeitos da Face, associate physician of the Hospital Municipal Infantil Menino Jesus, São Paulo, SP, Brazil.
2. Full memberof the SBCP, associate physician of the Hospital Santa Cruz, São Paulo, SP, Brazil.
3. Full member of the SBCP, associate physician of the Hospital dos Defeitos da Face, São Paulo, SP, Brazil.
4. Associate member of the SBCP, associate physician of the Hospital dos Defeitos da Face, São Paulo, SP, Brazil.
5. Associate member of the SBCP, plastic surgeon, São Paulo, SP, Brazil.
Correspondence to:
Guilherme Gurgel do Amaral Teles
Av. Moreira Guimarães, 699
São Paulo, SP, Brazil - CEP 04074-031
E-mail: guilhermeteles77@yahoo.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: April 9, 2011
Article accepted: July 23, 2012
This study was performed at the Hospital dos Defeitos da Face, São Paulo, SP, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter