

Original Article - Year 2012 - Volume 27 -
Mastopexy after massive weight loss: dermal suspension, parenchymal reshaping, and augmentation with autologous tissue
Mastopexia após perda ponderal maciça: suspensão dérmica, remodelação do parênquima e aumento com tecido autógeno
ABSTRACT
BACKGROUND: Patients who experience major weight loss may have pronounced breast ptosis, loss of projection of the higher pole, and excessive tissue in the lateral thorax. Rubin & Khachi described a mastopexy technique with dermal suspension and parenchymal remodeling associated with augmentation with autologous tissue. This technique treats the mammary deformity and the excessive tissue in the lateral thorax in a single surgery. In this study, we describe this surgical technique and demonstrate its reproducibility and the possible complications.
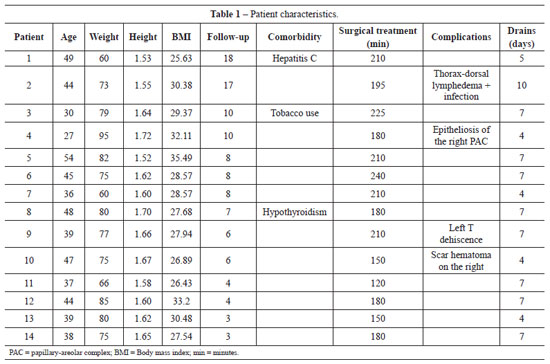
METHODS: From December 2008 to December 2009, surgery was performed using the technique described above on 14 patients with grade 2 and 3 deformities according to the Pittsburgh scale. The following data were analyzed: type of breast deformity, translocation of the papillary-areolar complex (PAC), dimension of the flaps used, surgical time, permanence time of the drain, and the incidence of complications.
RESULTS: The mean age of the patients was 41.21 ± 7.67 years and the mean body mass index was 29.30 ± 2.77. The follow-up period ranged from 3 months to 18 months, with a mean of 8 months. Among the 14 patients that underwent surgery, 4 patients (28.6%) had grade 3 deformities and 10 patients (71.4%), had grade 2 deformities. The mean translocation of the PAC was 6.38 cm, the mean dimensions of the flap were 25.21 cm × 6.92 cm, and the mean surgical time was 188.57 minutes. The drains remained for an average of 6.21 days. The following complications were observed: PAC epitheliosis, dehiscence of the T-junction, a small hematoma, and lateral thoracic lymphedema.
CONCLUSIONS: Mastopexy with dermal suspension, parenchyma remodeling, and augmentation with autologous tissue is a reproducible technique that can be performed quickly and has a low complication rate.
Keywords: Mammaplasty. Weight loss. Postoperative complications. Breast/surgery.
RESUMO
INTRODUÇÃO: Pacientes com perda ponderal significativa podem apresentar mamas com ptose acentuada, perda de projeção do polo superior e excesso de tecido na porção toracolateral. Rubin & Khachi descreveram técnica de mastopexia com suspensão dérmica e remodelação do parênquima associada a aumento com tecido autógeno, tratando a deformidade mamária e o excesso toracolateral em um só estágio. Neste trabalho, é ilustrada essa técnica cirúrgica e demonstradas sua reprodutibilidade e suas complicações.
MÉTODO: Foram operadas 14 pacientes com deformidade graus 2 e 3 pela Escala de Pittsburgh, no Hospital Estadual de Sapopemba (São Paulo, SP, Brasil), no período de dezembro de 2008 a dezembro de 2009, utilizando a técnica referida. Foram analisados os seguintes dados: tipos de deformidade das mamas, translocação do complexo areolopapilar (CAP), dimensões dos retalhos, tempo cirúrgico, tempo de permanência do dreno e incidência de complicações.
RESULTADOS: A média de idade das pacientes foi de 41,21 ± 7,67 anos e o índice de massa corporal médio foi de 29,30 ± 2,77. O tempo de seguimento das pacientes variou de 3 meses a 18 meses, com média de 8 meses. Dentre as 14 pacientes operadas, 4 (28,6%) apresentavam deformidade grau 3 e 10 (71,4%), grau 2. A média de translocação do CAP foi de 6,38 cm. As dimensões médias do retalho foram de 25,21 cm x 6,92 cm. O tempo cirúrgico médio foi de 188,57 minutos. Os drenos permaneceram, em média, por 6,21 dias. Foram observadas as seguintes complicações: epiteliose de CAP, deiscência na junção do T, hematoma pequeno e linfedema toracolateral.
CONCLUSÕES: A mastopexia com suspensão dérmica, remodelação do parênquima e aumento com tecido autólogo é uma técnica reprodutível, rápida e com baixo índice de complicações.
Palavras-chave: Mamoplastia. Perda de peso. Complicações pós-operatórias. Mama/cirurgia.
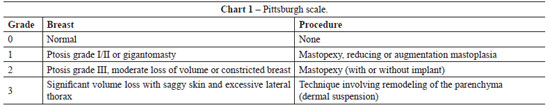
A growing number of women require mastoplasty after significant weight loss as a result of the increased number of bariatric surgeries for the treatment of obesity and its comorbidities1,2. According to the Pittsburgh scale3, 3 types of mammary deformities affect patients with massive weight loss: (1) light ptosis, suitable parenchyma, and no saggy skin; (2) ptosis grade II or III, saggy skin, light parenchyma atrophy, and no significant deviation of the papillary-areolar complex (PAC); and (3) a more pronounced deformity, with significant ptosis, saggy skin, intense parenchyma atrophy, and medial deviation of the PAC.
After rapid weight loss, qualitative changes in the skin and the loss of natural elasticity may cause the breasts to evolve with pronounced ptosis, sagginess, and loss of projection in the upper pole4. Furthermore, excessive unwanted tissue in the axillary and lateral thoracic region leads to deformity in the body shape and, consequently, patient dissatisfaction. In most cases, this deformity can be corrected by combining lipoaspiration with surgical resection5.
The aim of surgery is usually to achieve symmetry in the size and shape of both breasts and the PAC. Combining mastopexy with augmentation is required when both ptosis and loss of volume are present; this can be performed in stages or in 1 surgery. Traditionally, augmentation is performed using silicone implants, which is associated with the inherent risks of implant usage: capsular contracture, bleeding, ruptures, wrinkling, infection, mammography shadows, capsule calcification, and bad positioning, the latter of which is very common in patients with significant weight loss4. More recently, attention has turned to breast augmentation with autologous tissue, which would avoid complications related to the implant and would be economical because it does not require a prosthesis. This procedure is particularly relevant when the patient is reluctant to accept a prosthesis.
In this context, mastopexy with dermal suspension, parenchyma remodeling, and augmentation with autologous tissue, as described by Rubin & Khachi6, is a useful technique in the treatment of mammary deformities after significant weight loss. This method treats excessive skin in the lateral thorax and increases the mammary volume in a single surgery.
The aim of this study was to provide a detailed illustration of the surgical technique of mastopexy with dermal suspension, parenchyma remodeling, and augmentation with autologous tissue. Furthermore, we demonstrate the reproducibility of this technique and present the incidence of complications in our series of cases.
METHOD
We performed mastopexy with augmentation using autogenous tissue, dermal fixation, and remodeling of the mammary parenchyma on 14 patients from December 2008 to December 2009 (Table 1). All patients gave informed consent.
The mean age of patients at the time of surgery was 41.21 ± 7.67 years and the mean body mass index (BMI) was 29.30 ± 2.77.
The patients included in this study had not undergone previous mammary surgery and all had experienced significant weight loss after bariatric surgery. At least 1 year had passed since the gastroplasty and all patients had maintained a stable weight for at least 6 months.
In addition to breast repositioning, the patients sought to increase breast volume and to decrease the excessive tissue in the lateral thorax that caused changes in the shape of that region. A preoperative clinical evaluation was performed to exclude the possibility of malignant lesions in the breast.
The patients had grade 2 or 3 breast deformities according to the Pittsburgh scale3 in which mammary deformities after significant weight loss are classified from 0 to 3 according to gravity. This scale is defined as follows: (1) light ptosis, suitable parenchyma, and no saggy skin; (2) ptosis grade II or III, saggy skin, light parenchyma atrophy, but no significant deviation of the papillary-areolar complex (PAC); and (3) a more pronounced deformity, with significant ptosis, saggy skin, intense parenchyma atrophy, and medial deviation of the PAC (Chart 1).
We did not perform other surgeries combined with mastopexy. All surgeries were performed under general anesthesia with compressive tights and intermittent massage (Flebopress ®).
The following data were analyzed:
1. Type of breast deformity, according to the Pittsburgh scale;
2. PAC translocation (in cm);
3. Flap dimensions (length × width);
4. Surgical time;
5. Permanence time of the drain;
6. Incidence of complications.
Surgical Technique
The breasts were marked at the medial line starting from the sternal notch, the breast meridian, and the inframammary fold while the patients were standing upright (Figure 1). The point to which the PAC would be translocated (point A) was determined by crossing the line of the breast meridian with the inframammary fold. Points B and C were determined by the two-finger pinching maneuver7. Skin incisions were marked following the technique of rigid demarcation described by Pitanguy8 and Wise9, with a modification made only to the lateral prolongation of this demarcation. This prolongation corresponded exactly to the excessive tissue of the lateral thorax region, which was common in these patients and was used to promote breast augmentation. This was planned by the pinch test in order to identify the excessive skin and tissue to be included in the flap, without compromising the closure of the donor area. Intercostal perforators from the central and lower areas of the breast vascularize this entire bloc of tissue (lateral, central, and medial flap) as well as the PAC. The central and lower areas, which are responsible for vascularization, were limited to an 8 cm × 8 cm area in this study, which permitted adequate mobility and easy remodeling of the parenchyma.
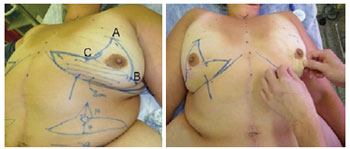
Figure 1 - In left, complete preoperative markings. The striped area corresponds to the vascular pedunculus of the flap. In right, the two-finger pinching maneuver and the definition of points B and C are shown.
The surgery was initiated by marking the areola with a 42-mm areolotome and de-epithelization of the dermal-adipose flaps (Figure 2).
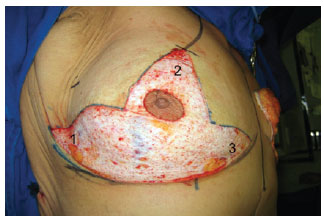
Figure 2 - De-epithelization of the dermoadipose flaps: 1) lateral flap, 2) central flap, and 3) medial flap.
The parenchyma flap was cranially separated with a thickness of 1.5 cm until the second rib, bilaterally, to allow for dermal suspension. The lateral and medial flaps were created while maintaining an area of 8 cm × 8 cm attached to the thorax to ensure nutrition to this bloc of tissue (Figure 3). The ends of the flaps were tested for viability by checking for bleeding from the dermal ends. If bleeding did not occur, resections were performed until the tissue was viable.
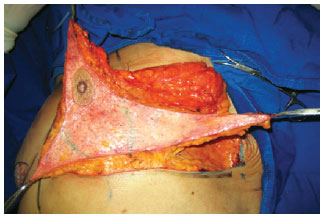
Figure 3 - Preparation of lateral, central, and medial flaps. An area of 8 × 8 cm (marked in green) was attached to the thorax to maintain the intercostal perforators.
Resections were performed to decrease the volume of the lateral, central, and medial flaps in patients with asymmetrical breasts until symmetry was achieved. Dermal suspension was performed by fixing the central flap in the upper-medial position to the periosteum of the second rib, using nylon 2-0 sutures. In addition, the lateral and medial flaps were fixed in a super-medial position as they approached the central flap. The fixation points were passed symmetrically between the right and left breast to provide similar projection of the upper pole of each breast. Parenchyma remodeling was performed by bringing the dermal ends of the central flap to the lateral and medial flaps, using vicryl® 2-0 single and/or continuous sutures. More stitches may be required in the dermis for the bloc to acquire a spherical shape, mimicking a mammary implant. This should be evaluated on a case-to-case basis (Figure 4).
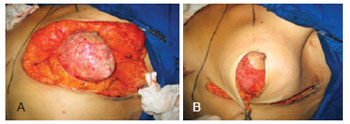
Figure 4 - In A, intraoperative appearance of mastopexy after dermal fixation and parenchyma remodeling. In B, the remodeled parenchyma covered with the cutaneous envelope.
Following dermal suspension and parenchyma remodeling, the structure was covered with the breast skin flap. Closure was initiated in 3 planes of the scar in an inverted T (subcutaneous with vicryl® 3-0, subdermal with monocryl® 3-0, and intradermal with monocryl® 4-0 sutures). When possible, the vertical component of the scar was maintained at 4-5 cm to avoid the development of postoperative pseudoptosis in the breasts.
The patient was placed in a sitting position on the surgical table to verify breast symmetry. If the breasts were symmetrical, the position of the PAC was planned and drawn using the 42-mm areolotome for molding. Because point A had been established during the preoperative phase, only small positioning adjustments were required for better symmetry and placement of the PAC in this latter phase. The areola was sutured in the subdermal and intradermal planes with monocryl® 5-0. The breasts were drained with a Portovac® 4.8, with the drain laterally exiting the horizontal scar. Simple wound management involved the use of a bandage and micropore tape attached to a bra, which was placed after the surgery.
The patients were discharged on the first day after surgery and returned for evaluation on the 4th, 7th, and 14th postoperative days and after 1, 3, and 6 months (Figures 5 and 6). The drains were removed when the drained fluid was less than 50 ml in 24 hours.
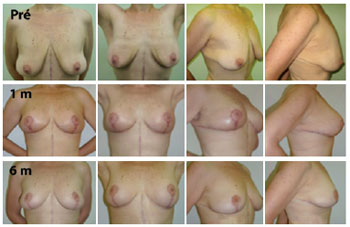
Figure 5 - Evolution up to 6 postoperative months after mastopexy for massive weight loss. Pré = preoperative; 1 m = 1 postoperative month; 6 m = 6 postoperative months.
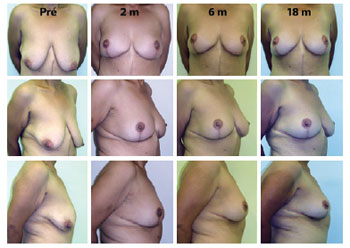
Figure 6 - Evolution up to 18 postoperative months after mastopexy for massive weight loss. Pré = preoperative; 1 m = 1 postoperative month; 6 m = 6 months postoperative; 18 m = 18 postoperative months.
RESULTS
Mastopexy and augmentation with autogenous tissue, dermal suspension, and parenchyma remodeling was performed in 14 patients that had experienced significant weight loss. The mean follow-up time was 8 ± 4.64 months, ranging from 3 to 18 months.
Among the 14 patients that underwent surgery, 4 (28.6%) had grade 3 deformity and 10 (71.4%) had grade 2 deformity according to the Pittsburgh scale. The distance between point A and the preoperatively measured PAC (PAC translocation) was 6.38 ± 1.63 cm. The mean dimensions of the flap were 25.21 ± 1.6 cm × 6.92 ± 1.24 cm. The mean surgical time was 188.57 ± 32.60 minutes, ranging from 120 minutes to 240 minutes. The mean permanence time of the drains was 6.21 ± 1.79 days.
With regard to complications, 1 case developed PAC epitheliosis (Figure 7) and 1 case had dehiscence in the T-junction (smaller than 1 cm in diameter). Furthermore, 1 case had a small hematoma in the horizontal scar and 1 case had lymphedema in the right lateral thorax (Figure 8). None of these patients required further surgeries. Adipose necrosis in the dermoadipose flaps was not detected in any of the patients after clinical evaluation.
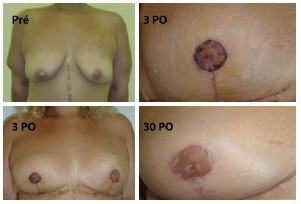
Figure 7 - A 27-year-old patient, weighing 95 kg and 1.72 m in height, with epitheliosis of the right PAC. Repair was complete after 21 days. Pré = preoperative; 3 PO = 3rd postoperative day; 30 PO = 30th postoperative day.
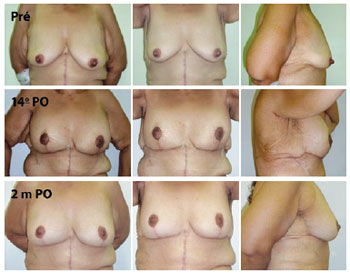
Figure 8 - A 44-year-old patient, weighing 73 kg and 1.55 m in height, 7 months after anchor abdominoplasty. The patient developed significant lymphedema in the right thorax region. Complete regression of the lymphedema occurred by 2 postoperative months. Pré = preoperative; 14 PO = 14th postoperative day; 2 m PO = 2 postoperative months.
DISCUSSION
According to Ferreira10, patients who underwent mastoplasty had increased self-esteem and, consequently, a better quality of life.
As previously discussed, mammary deformities in patients with weight loss of 50% to 70% generally result in serious ptosis, parenchyma atrophy, saggy skin, PAC medialization, and grade 2-3 deformities in the Pittsburgh scale3. In these cases, a combination of mastopexy and augmentation is indicated. Mastopexy techniques with augmentation using implants were described for ptotic and hypoplastic breasts11,12, but they are widely used in our field for patients with significant weight loss. The surgical procedure is complex in these cases and carries a high risk of complications including exposure of the implant, bad placement, infection, loss of the PAC, skin flap necrosis, an unaesthetic scar, and uncertainty of the final position of the areola4. Furthermore, these procedures are associated with the inherent risks of implant use3.
Augmentation mastoplasty with autologous tissue was initially described by Ribeiro13 in 1975. This technique uses the excessive tissue of the lower and center pole of the breast to prepare a flap with an inferior pedunculus that will function as a natural implant and fill in the upper pole of the breast when fixed under the PAC14. Subsequently, Graff & Biggs15-17 described several modifications to the technique. However, these techniques normally are not used in patients with massive weight loss.
Mastopexy with dermal fixation and parenchyma remodeling associated with augmentation with autologous tissue was described by Rubin & Khachi6. This technique uses the excessive tissue of the lateral thorax of the breast, which is common in patients with significant weight loss. In addition to promoting breast augmentation with autologous tissue, this technique treats the excessive skin in the lateral thorax that normally causes deformity in this region. Dermal fixation of the center flap to the periosteum of the second rib theoretically maintains the filling of the upper pole of the breast for a longer period and parenchyma remodeling for the autologous flaps using stitches in the dermis gives better shape to the breasts.
In this study, all cases were type 2 (71.4%) or type 3 (28.6%) according to the Pittsburgh scale, with atrophy of the parenchyma, significant ptosis, and a PAC translocation of 6.38 ± 1.63 cm. Although the flaps used for augmentation were large (25.21 cm × 6.92 cm), the perforant vessels of the intercostals in the pedunculus (an 8 cm × 8 cm area) in the central and lower portion of the breast were adequate for irrigation because adipose or PAC necrosis, which would suggest vascular compromise, was not observed in the postoperative period. The mean surgical time of 3 hours (188.57 minutes) is similar to other mastoplasty techniques, even though this technique is new to our field.
With regard to complications, we observed 1 case of PAC epitheliosis, 1 case of T dehiscence, 1 case with a small hematoma in the horizontal scar, and 1 right thorax lymphedema among 14 patients. None of these cases required another surgery. Only the lymphedema had a slow evolution, with clinical regression at 2 months and manual lymphatic drainage performed twice a week. After this episode, we made the lateral flaps a little bit thinner and left a layer of fat over the muscle of the lateral thorax wall in an attempt to preserve a part of the lymphatic system. Episodes of lymphedema did not occur after these changes were made. The incidence of complications associated with this technique was low, similar to other studies in the literature6,7.
The horizontal scar from this procedure is longer than in other mastoplasty techniques because of lateral prolongation, but this did not cause complaints from any of the patients during the postoperative period. Although this is part of the subjective criteria, all patients who underwent this procedure were satisfied with the results.
CONCLUSIONS
Mastopexy with dermal suspension, parenchyma remodeling, and augmentation with autologous tissue is a reproducible technique that can be performed quickly and has a low complication rate.
REFERENCES
1. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339-52.
2. Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316-23.
3. Song AY, Jean DR, Hurwitz DJ, Fernstein MH, Scott JA, Rubin JP. A classification of contour deformities after bariatric weight loss: the Pittsburgh Rating Scale. Plast Reconstr Surg. 2005;116(5):1535-46.
4. Thornton DJ, Fourie LR. Autologous augmentation-mastopexy after bariatric surgery: waste not want not! Aesthetic Plast Surg. 2010;34(4):519-24.
5. Aly AS, Cram AE, Heddens C. Truncal body contouring surgery in the massive weight loss patient. Clin Plast Surg. 2004;31(4):611-24.
6. Rubin JP, Khachi G. Mastopexy after massive weight loss: dermal suspension and selective auto-augmentation. Clin Plast Surg. 2008;35(1):123-9.
7. Modolin M, Cintra W Jr, Silva MM, Ribeiro L, Gemperli R, Ferreira MC. Mammaplasty with inferior pedicle flap after massive weight loss. Aesthetic Plast Surg. 2010;34(5):596-602.
8. Pitanguy I. Surgical treatment of breast hypertrophy. Br J Plast Surg. 1967;20(1):78-85.
9. Wise RJ. A preliminary report on a method of planning the mammaplasty. Plast Reconstr Surg. 1956;17(5):367-75.
10. Ferreira MC. Evaluation of results in aesthetic plastic surgery: preliminary observations on mammaplasty. Plast Reconstr Surg. 2000;106(7):1630-9.
11. Gonzales-Ulloa M. Correction of hypotrophy of the breast by means of exogenous material. Plast Reconstr Surg Transplant Bull. 1960;25(1):15-26.
12. Regnault P. The hypoplastic and ptotic breast: a combined operation with prosthetic augmentation. Plast Reconstr Surg. 1966;37(1):31-7.
13. Ribeiro L. A new technique for reduction mammaplasty. Plast Reconstr Surg. 1975;55(3):330-4.
14. Ribeiro L, Accorsi A Jr, Buss A, Marcal-Pessoa M. Creation and evolution of 30 years of the inferior pedicle in reduction mammaplasties. Plast Reconstr Surg. 2002;110(3):960-70.
15. Graf R, Biggs TM, Steely RL. Breast shape: a technique for better upper pole fullness. Aesthetic Plast Surg. 2000;24(5):348-52.
16. Graf R, Biggs TM. In search of better shape in mastopexy and reduction mammoplasty. Plast Reconstr Surg. 2002;110(1):309-22.
17. Graf R, Biggs TM. Mastopexy with a pectoralis loop. In: Spear S, ed. Surgery of the breast. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 1008-39.
1. Full member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery) - SBCP, Plastic surgeon collaborator for the ex-obese group at the Hospital Estadual de Sapopemba (Sapopemba State Hospital), São Paulo, SP, Brazil.
2. Full member of the SBCP, Coordinator of the ex-obese group at the Hospital Estadual de Sapopemba (Sapopemba State Hospital), São Paulo, SP, Brazil.
3. Specialist member of the SBCP, Plastic surgeon collaborator for the ex-obese group at the Hospital Estadual de Sapopemba (Sapopemba State Hospital), São Paulo, SP, Brazil.
4. Specialist member of the SBCP, Coordinator of the ex-obese group at the Hospital Estadual de Sapopemba (Sapopemba State Hospital), São Paulo, SP, Brazil.
5. Full member of the SBCP, Associate Professor of the Faculdade de Medicina da Universidade de São Paulo (Faculty of Medicine at the University of São Paulo) - FMUSP, São Paulo, SP, Brazil.
6. Full member of SBCP, Professor of Plastic Surgery at the FMUSP, Chief of the Plastic Surgery service at the Hospital of FMUSP, São Paulo, SP, Brazil.
Correspondence to:
Patricia Yuko Hiraki
Rua Oscar Freire, 1702 - ap. 71 - Cerqueira César
São Paulo, SP, Brazil - CEP 05409-011
E-mail: patyuko@hotmail.com
Article submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: March 2, 2012
Article accepted: May 24, 2012
Study performed at the Hospital Estadual de Sapopemba (Sapopemba State Hospital), São Paulo, SP, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter