

Original Article - Year 2012 - Volume 27 -
The role of Research Ethics Committees
O papel dos comitês de ética em pesquisa
ABSTRACT
Research ethics committees are responsible for the ethical evaluation of research projects. Moreover, they must inform and educate their members and the community on their role in protecting the society. For some researchers, ethics committees are a target of criticism, although they are currently essential in the field of medical research and publications. The present study uses a documental analysis to describe the functioning of the research ethics committee system by focusing on the National Research Ethics Committee, Sarah Network of Rehabilitation Hospitals.
Keywords: Ethics. Research. Ethics committees.
RESUMO
Os comitês de ética em pesquisa são responsáveis pela avaliação ética dos projetos de pesquisa; ademais, devem informar e educar seus membros e a comunidade quanto a sua função no controle social. Para alguns pesquisadores, os comitês de ética são alvo de muitas críticas; todavia, na atualidade, são imprescindíveis no campo das pesquisas e publicações médicas. Os autores descreveram por meio de análise documental o funcionamento do sistema de comitês de ética em pesquisa, da Comissão Nacional de Ética em Pesquisa e da Rede Sarah de Hospitais de Reabilitação.
Palavras-chave: Ética. Pesquisa. Comissão de ética.
The ethics of health care professionals should include all aspects of scientific assistance, performing research, and scientific publications1. The organization of committees for ethics review in research began in the 1960s.
In Brazil, this system was created through a resolution adopted in 1988 by the Conselho Nacional de Saúde [National Council of Health (CNS)], a social control agency linked to the Ministry of Health. The system was later revised by Resolution 196/96 2, which defined the guidelines for the creation and consolidation of the Brazilian organization for research ethics review, namely, the Institutional Review Board (IRB)/Comissão Nacional de Ética em Pesquisa - National Research Ethics Committee (IRB/CONEP) system. In addition to this resolution, the CNS put forth several complementary resolutions addressing the regulation and accreditation of IRB, standard rules for special areas, foreign cooperation, human genetics and reproduction with native people, multicenter projects, and storage and use of biological material3.
According to Resolution 196/96 2, research is defined as "class of activities with the objective of developing or contributing to generalizable knowledge. Generalizable knowledge consists of theories, relations, principles, or accumulation of information on which the project is based and that can be corroborated by accepted scientific methods, observations, and conclusions. Research involving human beings is that which, individually or collectively, directly or indirectly, totally or partially, involves humans, including the management of information or material". The presentation of research to the community is achieved through congresses, seminars, and scientific publications. According to Pearn4, the publication of a scientific paper is an ethical obligation, and is an important criterion for the evaluation of scientific results. In a study by Sardenberg et al.5 performed in 1999, the norms related to ethics in research studies published by 139 Brazilian scientific journals were evaluated. It was observed that 79.1% studies did not reference ethical rules, 12% required prior approval of the Ethics Commission, and the remaining reports mentioned the Declaration of Helsinki, asked for consent, or did not have established norms. In that report, the Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery (BJPS) was included among those that did not require ethical references, probably because it implied that the study was performed according to norms, rules, and ethical principles as recommended by the Brazilian laws. However, the field of research and publications has become vast and diverse, and the editorial policies of the journals require constant evidence of evaluation by institutional ethics committees. Currently, according to the policy of BJPS6 and those of other national journals, such as Arquivos Brasileiros de Cardiologia (Brazilian Archives of Cardiology)7, the authors should state that the research was approved by the Research Ethics Committee of their institution, in line with the Declaration of Helsinki6, reviewed in 2000, and follows the guidelines of Resolution 196/96 2. Moreover, the authors are required to submit the number of the protocol obtained after application in the site of the Plataforma Brasil (Brazil Platform): http://www.cometica.ufpr.br/ORIENTACOES%20BASICAS%20PLATAFORMA%20BRASIL.pdf.
Ethics committees are characterized by the multidisciplinary training of their members, and evaluate the research at all stages of studies involving humans, from the design of a project until its final report and publication. The activities of the ethics committees, the CONEP and CNS, in educating and supervising research studies ensures the preservation of human rights as a prerogative of all members of society. We should not ignore the fact that the existence of an ethics committee does not mean that the ethical principles of research have been achieved. The system requires specific criteria for evaluation and operational capacity.
The aim of this paper is to contextualize the role of IRBs and their importance in publication in scientific journals.
IRB/CONEP SYSTEM
IRB is an institutional organization whose involvement must be internally regulated with regard to the analysis of research protocols of an institution or as a co-participant. There are situations in which the IRB must exercise its function, for example by designation of the CONEP, for the analysis of a project led by a researcher who is linked to an institution that does not have a IRB. In multicenter studies, the IRBs of all the participant institutions must follow the research protocol6.
To create an IRB, an institution must apply for registration and provide the CONEP with the following documents: an act of committee creation, including a description of the mission and general activities of the proposing institution; and data on the members, the institution, and the coordinator, which are the representatives of the civil society organized by the users. The instructions for the formation and functioning of an IRB are contained in the manual of the committee formulated by CONEP6, which can be downloaded from the CNS website (Form)8. It is worth mentioning that institutional involvement is a mandatory for the creation and functioning of an IRB, as the institution covers all the costs.
The organization of an IRB is an interdisciplinary process, and must comply with the norms of Resolution 196/96 2. At the most, 50% of the members of the IRB should belong to the same professional category, and the participation of individuals that are not involved in research must also be ensured. In other words, it is not a committee of researchers, but rather a group of individuals representative of the society. The aim of IRBs is to analyze the aspects of biomedical research protocols that are related to research subjects, and the importance and relevance of the research. The protocols must also be evaluated according to the effort, resources, and time spent. The IRB is also responsible for monitoring the progress of research projects.
IRB FUNCTIONING
The members of the IRB, as well as researchers, must be enrolled in Plataforma Brazil, national and unified database for record of research involving humans of the system IRB/CONEP. It allows searches to be accompanied in its different stages, from submission to approval by the IRB and the CONEP, when necessary; also allowing the monitoring of the field phase and the sending of partial and final reports. The researcher must register on the platform describing preliminary information, the study area, study design/financial support, study design and other information, finalize and click the "send to the IRB project." The projects come to the office designated by the IRB, which distributes to its members and the coordinator, and all receive e-mail notice of matters on the platform. The reporters get the project and after considering issuing its opinion. After they are evaluated by the recorder, the projects are subjected to evaluation by other IRB members during monthly or fortnightly meetings, depending on the number of projects that require analysis. The meetings must include more than half of the collegiate to deliberate and/or approve research projects, which must be registered after receiving the signatures of all the members present. The submission of a protocol to IRB is independent of the level of research, and can consist of a graduate student project, a doctoral thesis, or a scientific initiation, and it can be an academic or applied project, as long as it is included in the definition of "research involving humans".
The documents required for the analysis of research projects are listed in Chart 1. In the absence of any of these documents, the researcher must include a communication to the coordinator of the IRB explaining the reason for this omission. The project must be prepared using Microsoft Word and all the documentation must be scanned and sent in digital format on a CD or using the website of the CNS (Plataforma Brasil)9. The cover page is the main document and it gives the project legal validity, identifies the research project, the responsible researcher, the proposing institution, the principal investigator, the sponsor, and the co-participants8. In cases in which the researcher or the proposing institution does not have an IRB, a request is sent to CONEP to obtain an IRB indication more suitable for the proposed project, or the project can also be directly sent to CONEP, which will assign the project to an IRB in the proximity of the researcher. The Informed Consent Form (ICF) is formulated for a voluntary decision by an independent and capable individual, made after a process of informed deliberation, and is aimed at obtaining approval for a specific treatment or experimental protocol, with awareness of its nature, consequences, and risks10. There are special situations in which the ICF may be dispensed (CNS Resolution no 196/96-IV.3.c); in these cases, it must be replaced by a justification explaining why it is not necessary or the reasons why it could not be obtained, and the IRB shall assess the relevance. All data collection instruments must be clearly described, because the objective of the ICF (Annex 1) is to ensure that the research subject has the right to refuse answering questions that may cause embarrassment of any nature. It is important that the IRB is aware of the questionnaires used, as sometimes changes are required to make the research tools more ethically appropriate and less invasive for the individual's privacy. Documents with a letterhead should not be used for data collection. The principal investigator and participating researchers should submit their resumes in the Lattes Platform, attaching only the first page containing the Lattes address. The resume must be updated at least 90 days prior to the date of valuation.
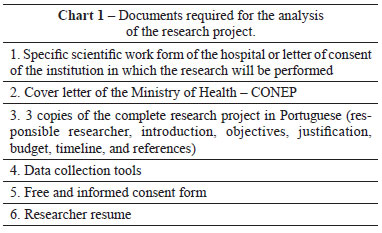
The members of the IRB prepare a report that includes the ethical and methodological faults, reactions, and adverse effects of the proposed research; assessment of risks and benefits; study inclusion and exclusion criteria; privacy and confidentiality of the research subjects; and the names of the IRB members. The members prepare the consolidated report, and designate the project as "approved", "approved with recommendations", "pending," or "not approved".
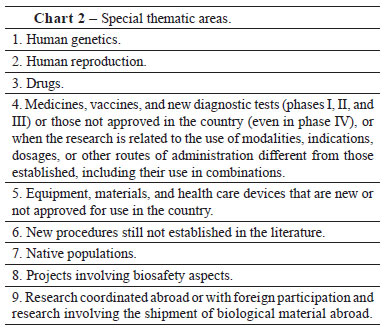
The amendments represent any proposed changes made to the objectives and methodology of the initial project. After approval, the project must be directed to CONEP for revision of special thematic areas (Chart 2), or it can be withdrawn, if the investigator does not provide the requested answers to questions; canceled, when there is an interruption before the start of the study; suspended, when the interruption occurs during the course of the research; or terminated, when finalized after completion of the steps proposed. Moreover, the investigator must submit the partial and final research report. According to Freitas and Hossne1, the special thematic areas were designated as such because they often bring to light important ethical dilemmas, which frequently lack consensus in the society and have major repercussions for the research subject. For this reason, after the evaluation of the project by the institutional IRB, the final assessment is performed by the CONEP.
There are currently 628 IRBs registered in CONEP, and they must meet the criteria for registration and renewal, submit monthly reports, and evaluate at least 12 projects per year.
Several resolutions are available on the CONEP website concerning the regulations applicable to linked or proposed institutions, such as the electoral process and other deliberations related to research involving humans. To accommodate the growing demands and improve the system operating conditions, IRB/CONEP created the Sistema Nacional de Informação sobre Ética em Pesquisa [National System of Information on Research Ethics (SISNEP)], which enabled the registration of research projects, although not all the IRBs were using it. In an attempt to broaden this system, in January 2012, the IRB/CONEP implemented the Plataforma Brasil9, a unified national registry for research involving humans. The CONEP is organizing training workshops for system implementation. The coordinators of the IRBs of Brasilia met on September 21 and 22, 2011 in the auditorium of the CNS of the Ministry of Health for the presentation of this system and for orientation on the operation of the system.
The CNS Resolution 196/96 2, which establishes the guidelines and regulatory norms for research projects involving humans, was released to the public on 10/11/2011. The rules of this Resolution have been in effect since 1996. It was a moment of great importance when the possibility of submitting suggestions became a reality.
CONEP FUNCTIONING
The CONEP is responsible for the coordination of the IRB networks of several types of institutions that to some extent work with human research, forming the System IRB/CONEP8. It is a consultant agency of the Ministry of Health and other agencies that constitute the Unified Health System (SUS) in Brazil. The IRB sends the final copy of research protocols to the CONEP for consideration (according to standard norms and flowcharts), with possible modifications as requested by the IRB. Possibly, the research protocol should be signed on all pages and should include a consolidated written opinion if consensus was not achieved on a specific issue and if further evaluation by the CONEP is necessitated, according to IRB norms. In addition, notification of adverse events should also be sent to the CONEP after consideration and immediate measures taken by the researcher and others. The IRB must inform the CONEP in case of changes in its composition, the mandate of its members on the election of new members of the college or choose new coordinator, the specific queries about ethics in research involving human subjects and suggestions for improvement and adequacy of the system and standards.
EXPERIENCE IN SARAH NETWORK OF REHABILITATION HOSPITALS
The IRB at Sarah Network of Rehabilitation Hospitals is a collegiate organ established according to the regulations and structure of Resolution 196/96 of the CNS/CONEP.
The IRB was established in 2001 with the main objective of promoting the application of principles on the basis of the ethics of human rights in all research involving humans. The decisions of the IRB are independent of special, institutional, or personal interests.
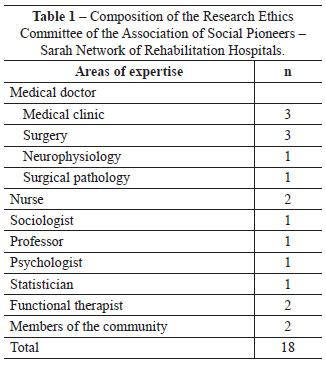
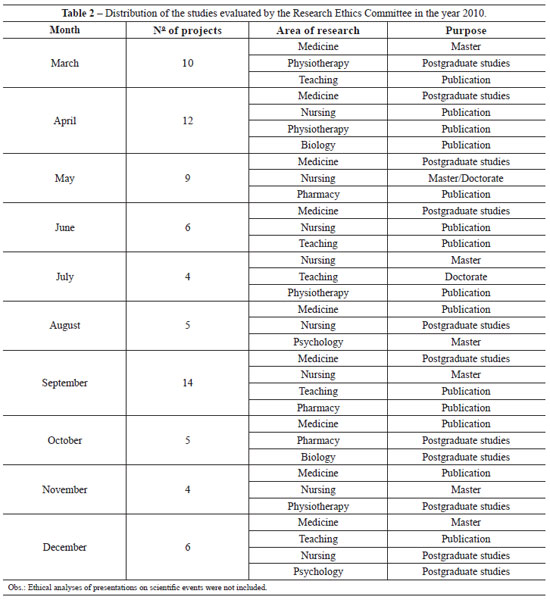
The collegiate is comprises 1 coordinator, 10 members, and 7 substitutes. It is committed to the fair representation of gender and occupation among its members (Tables 1 and 2). The IRB meets monthly to analyze proposals of research projects involving humans, with occasional meetings for conceptual discussion of ethics in research and the improvement of the identity of the IRB.
The research projects are presented for consideration by the IRB before the phase that involves subject participation, that is, before the data collection step. The performance of the analysis and preparation of the report occur within no more than 30 days.
The main problems presented by research projects are related to the ICF (language and lack of clarification of risks and benefits, among others), projects in English, non-inclusion of budget and schedule, imprecise definition of sample and data collection instruments, and methodological errors (in the description of the technique).
FINAL CONSIDERATIONS
In the formation of an IRB, it is important to ensure that its members are not considered representatives of a group of interest, and avoid adherence to certain religious beliefs or corporate institutions. The IRB is not the place for negotiation of corporate interests; the objective should be to evaluate the impact of research on the welfare of the population.
Considerable effort has been made to implement programs of orientation and training of CONEP members, the Executive Secretary, and institutional IRBs. The efficiency of the system can be valued for its protective role, considering several factors including the subjects, investigators, sponsors, and the government itself. However, the system has still been criticized as slow because of the numerous procedures and documents required.
Another aspect is related to the difficulty in the supervision and control of the projects authorized. In several countries, the abuse of research subjects has been reported. Fortunately, Brazil, as a result of the role played by the IRB/CONEP system, has been spared by the international press, which has exposed the lack of standards rules and structures of social control that led to the exploitation of vulnerable populations11.
We emphasize the essential role of IRBs in publicizing the ethical rules of research with humans, educating researchers, and establishing the correct way to design and carry out a research project12. However, despite these efforts, there are still difficulties and priorities that need to be addressed, in particular with regard to deadlines and the procedure required for the approval of projects, which currently seem to be minimized with the creation of a computerized system called Plataforma Brasil.
REFERENCES
1. Freitas CBD, Hossne WS. O papel dos comitês de ética em pesquisa na proteção do ser humano. Rev Bioética. 2002;10(2):129-46.
2. Brasil. Conselho Nacional de Saúde, Ministério da Saúde. Diretrizes e normas regulamentadoras da pesquisa em seres humanos: Resolução 196,1996. Disponível em: conselho.saude.gov.br/resolucoes/reso_96.htm - 113k. Acesso em: 30/9/2011.
3. Brasil. Conselho Nacional de Saúde, Ministério da Saúde. Disponível em: conselho.saude.gov.br. Acessado em: 28/9/2011.
4. Pearn J. Publication: an ethical imperative. BMJ. 1995;310(6990):1313-5.
5. Sardenberg T, Müller SS, Pereira HR, Oliveira RA, Hossne WS. Análise dos aspectos éticos da pesquisa em seres humanos contidos nas instruções aos autores de 139 revistas científicas brasileiras. Rev Assoc Med Bras. 1999;45(4):295-302.
6. Revista da Sociedade Brasileira de Cirurgia Plástica. Normas aos autores. Disponível em: http://www.rbcp.org.br/conteudo.asp?pag=1. Acesso em: 20/9/2011.
7. Arquivos Brasileiros de Cardiologia. Normas aos autores. Disponível em: http://www.arquivosonline.com.br. Acesso em: 20/9/2011.
8. Brasil. Comissão Nacional de Ética em Pesquisa. Formulários. Disponível em: http://conselho.saude.gov.br/web_comissoes/conep/index.html. Acesso em: 28/9/2011.
9. Brasil. Comissão Nacional de Ética em Pesquisa. Plataforma Brasil. Disponível em: www.saude.gov.br/plataformabrasil. Acesso em: 21/9/2011.
10. Saunders CM, Baum M, Houghton J. Consent, research and the doctor-patient relationship. In: Gillon R, ed. Principles of health care ethics. London: John Wiley & Sons; 1994. p. 457-70.
11. Brasil. Conselho Nacional de Saúde. Consulta pública da Resolução 196/96. Disponível em: http://portal.saude.gov.br/portal/saude/Gestor/area.cfm?id_area=1253. Acesso em: 22/9/2011.
12. Muñoz DR. Problemas e soluções no desenvolvimento de um CEP. Cad Ética Pesquisa. 2005;15(5):9-11.
1. Full Member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery) - SBCP, Research Ethics Committee Coordinator of the Associação das Pioneiras Sociais - Rede Sarah de Hospitais de Reabilitação (Social Pioneers Association - Sarah Network of Rehabilitation Hospitals), Bioethics Specialist and Master, President of the Regional DF for the biennium 2012-2013, Plastic Surgeon at Hospital Sarah Brasília (Sarah Brasília Hospital), Brasília, DF, Brazil.
2. Medical Doctor of the Força Aérea Brasileira (Brazilian Air Force), Brasília, DF, Brazil.
3. Senior Nurse at the Rede Sarah de Hospitais de Reabilitação (Sarah Network of Rehabilitation Hospitals), Specialist in the Surgical Center, Center of Material and Occupational Nursing, Brasília, DF, Brazil.
Kátia Torres Batista
SMHS Quadra 301 - Bloco B - Nº 45 - 3º andar - Asa Sul
Brasília, DF, Brazil - IRB 70330-150
E-mail: comiteeticapesquisa@sarah.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: August 11, 2011
Article accepted: January 10, 2012
Study conducted at the Associação das Pioneiras Sociais - Rede Sarah de Hospitais de Reabilitação (Social Pioneers Association - Sarah Network of the Rehabilitation Hospitals), Brasília, DF, Brazil.



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter