ABSTRACT
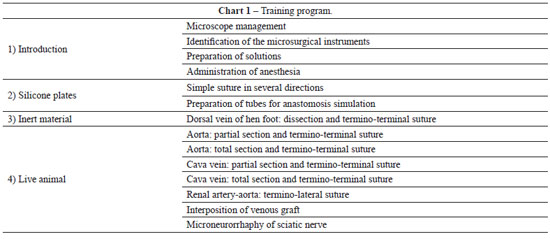
Microsurgery is a technique in which the surgery is performed under optical magnification in vessels with a diameter of less than 3 mm. In 1960, Jacobson and Suarez first used the term "microsurgery" to describe the experimental anastomosis of vessels with a caliber between 1 and 2 mm, which is considered as the origin of the modern microvascular practice. Since then, several types of microvascular tissue transfer techniques for the repair of large body defects have been developed and published, accompanied by significant advances in optical imaging technologies and instrument design. Despite this technical progress, laboratory practice is essential and enables the surgeon to acquire the ability to master the microanastomosis technique. The present study describes the microsurgery training program of the Laboratory of Experimental Microsurgery of the National Cancer Institute (Rio de Janeiro, RJ, Brazil).
Keywords: Microsurgery. Reconstructive surgical procedures. Training. Plastic surgery.