

Original Article - Year 2012 - Volume 27 -
Breast reconstruction with the latissimus dorsi muscle flap and alloplastic materials: analysis of results and proposal of a new technique to cover the implant
Reconstrução mamária com retalho do músculo grande dorsal e materiais aloplásticos: análise de resultados e proposta de nova tática para cobertura do implante
ABSTRACT
BACKGROUND: Breast reconstruction is distinct among plastic surgery techniques in that it requires the integration of several medical specialties as well as coordination with the patient. The aim of the present study was to analyze the results of breast reconstruction with the latissimus dorsi myocutaneous flap, and propose a strategy for better coverage and positioning of the implant.
METHODS: The study included 19 patients who underwent surgery between June 2006 and June 2009. Bilateral surgery was performed in 2 patients, and a total of 21 reconstructions were analyzed. The patients filled out a questionnaire on the aesthetic and functional aspects of the reconstruction. The complications, problems, and aesthetic improvement associated with the use of implants placed under a double layer of muscle were assessed.
RESULTS: A low rate of complications was reported, and only one case required a new surgical intervention to reposition the implant in relation to the inframammary crease. After the procedure, 94% of the patients reported that their expectations had been met, 64% reported no functional limitations, and 18% reported mild limitations. The placement of implants (prostheses or expanders) under the pectoralis major muscle, using the latissimus dorsi muscle flap to cover the implant improved the breast contour by softening the inframammary crease and positioning the implants in the upper and medial quadrants of the new breasts.
CONCLUSIONS: Breast reconstruction using silicone implants and the latissimus dorsi muscle flap can have excellent outcomes, with low rates of complications. Placing the implant under a double layer of muscle improves the harmony of the upper quadrants during breast reconstruction.
Keywords: Mammaplasty. Breast/surgery. Breast neoplasms. Surgical flaps.
RESUMO
INTRODUÇÃO: A reconstrução mamária ocupa lugar de destaque na cirurgia plástica e exige maior doação, entrosamento e confiança entre as especialidades médicas envolvidas e a paciente. O objetivo deste trabalho é analisar os resultados das reconstruções mamárias com o músculo grande dorsal e propor uma tática para melhor cobertura e posicionamento do implante.
MÉTODO: Dezenove pacientes, 2 delas submetidas a cirurgia bilateral, totalizando 21 reconstruções, foram operadas entre junho de 2006 e junho de 2009. As pacientes foram analisadas por meio de questionário sobre aspectos estéticos e funcionais da reconstrução. Foram estudadas intercorrências, complicações e melhora estética com uso do implante sob dupla camada muscular.
RESULTADOS: O índice de complicações foi pequeno, e em apenas um caso houve necessidade de reabordagem cirúrgica para reposicionar o implante em relação ao sulco submamário. Após o procedimento, 94% das pacientes afirmaram que tiveram suas expectativas atingidas, 64% não referiram limitações funcionais e 18% referiram limitações leves. O fato de colocar os implantes (próteses ou expansores) sob o músculo peitoral maior e cobrir o conjunto com o retalho do músculo dorsal melhora o contorno, pois abole ou suaviza as dobras e a aparência dos implantes nos quadrantes superiores e mediais das neomamas.
CONCLUSÕES: As reconstruções mamárias com retalho do músculo grande dorsal associado a implantes de silicone podem oferecer excelentes resultados, com baixos índices de complicações. A colocação do implante sob dupla camada muscular proporciona a obtenção de mais harmonia nos quadrantes superiores das neomamas.
Palavras-chave: Mamoplastia. Mama/cirurgia. Neoplasias da mama. Retalhos cirúrgicos.
The fight against breast cancer dates back to 1889, when Halsted1 performed curative radical mastectomies without considering the possibility of breast reconstruction. For many years, thousands of mutilated patients were left to cope with various aesthetic, functional, and psychological alterations.
Currently, breast cancer is the main cause of cancer-related death among women. Breast cancer records show a progressive increase in its incidence during the period between 1950 to 1990 owing to the success of prevention campaigns and the development of diagnostic methods. Approximately 50.71 new cases are expected to be diagnosed per 100,000 inhabitants in Brazil per year, according to the 2008 estimations by the National Institute of Cancer (INCA).
Recent studies demonstrate a significant increase in survival after mastectomy, which is often associated with radiotherapy, chemotherapy, and endocrine therapy2,3.
Until recently, breast reconstruction was considered of secondary importance in treatment planning. However, plastic surgeons insisted that it be given importance. The greater awareness of breast reconstruction as part of breast cancer treatment, and the increased demands of patients both in the public and private sectors have resulted in the generation of a new group of patients that require the work and dedication of plastic surgeons familiar with the current reconstruction techniques.
Breast reconstruction is essential to improve the quality of life of patients affected by breast cancer. The development of methods for early diagnosis and supporting treatments as well as a better understanding of the disease by mastologists made them seek in plastic surgery a support for the integral treatment of patients4. All these factors evolved to the current understanding that immediate breast reconstruction is necessary, especially in light of the good outcomes that can be achieved with the current techniques. In addition, the development of more suitable alloplastic materials (prostheses and expanders) of better quality has provided the plastic surgeon with more options to improve the tolerability of breast implants5.
Breast reconstruction is used for the repair of small defects (tumorectomy, segmentectomy, and quadrantectomy) and also after mastectomy. In cases of wide quadrantectomy or mastectomy, the alloplastic material can be associated with several alternatives in terms of surgical flaps.
Among the possible flaps at distance, the following are highlighted: the rectus abdominis myocutaneous flap (TRAM), described by Drever6 in 1977 and modified by Hartrampf et al.7 and Gandolfo8, in 1982, and the latissimus dorsi muscle flap (LDMF), described by Tansini9 and modified by Bostwick et al.10, in 1978. The LDMF is frequently associated with silicone prostheses or expanders, with the purpose of increasing the volume and shaping the breast cone. The use of permanent, two-compartment expanders is more recent.
After planning the immediate breast reconstruction based on preoperative staging criteria, it is important to predict the need for postoperative radiotherapy. In advanced tumors, in which radiotherapy is often required, it is desirable to perform the reconstruction with autologous tissue if available. However, when the inclusion of alloplastic material is required, supporting information can be found in the literature even in face of high indexes of current capsular contracture11. Reconstructions with LDMF and alloplastic materials have shown better results than those performed only with subpectoral expanders owing to the inclusion of increased amounts of tissue12.
The aim of the present study was to analyze the results of breast reconstructions performed with pedicled LDMF and silicone implants from a technical viewpoint and by using a subjective assessment of patients with specific issues. In addition, we analyzed the complications and problems observed in the present case selection and proposed a new surgical strategy based on the use of a double muscle layer for breast reconstruction with LDMF and implants.
METHODS
Of a total of 80 breast reconstructions performed between July 2006 and June 2009, 19 patients underwent breast reconstruction with pedicled LDMF associated with silicone prosthesis or permanent expanders. A total of 21 reconstructions (2 cases with bilateral reconstruction) were analyzed (Figure 1).
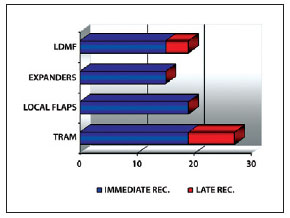
Figure 1 - Distribution of the samples with regard to the type of reconstruction. REC. = recovery; LDMF = latissimus dorsi muscle flap; TRAM = myocutaneous flap of the rectus abdominis.
The following criteria were applied for the use of LDMF: absence of abdominal donor area, presence of risk factors considered to be contraindications for TRAM (classically defined by Hartrampf et al.7 as tabagism, hypertension, diabetes, obesity, and depression), impossibility of reconstruction with local flaps, and patient agreement.
The patients received information concerning the technical details of the surgery, its limitations, and other reconstruction possibilities, if any, including late breast reconstruction.
All patients were operated under general anesthesia by the senior author.
The skin island arrangement and its dimensions, which were defined with the patients in a supine position, were mostly horizontal (Figure 2).
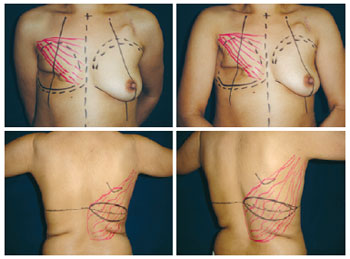
Figure 2 - Planning of the reconstruction with the latissimus dorsi muscle flap and prosthesis using a horizontal skin island after mastectomy.
A skin island in an oblique arrangement was used in only 2 cases of late reconstructions, which could not be achieved with the horizontal island. In 2 cases, only a muscle flap was used because the skin and nipple areolar complex (NAC) were preserved after mastectomy.
During the surgery, the LDMF pedicle was identified while the patient was in the supine position after resection of the parts by the mastologist or the dissection of the new breast site in cases of late reconstructions. A tunnel was then delineated for the dorsum, and the patient was placed in lateral decubitus, contralaterally to the unilateral mastectomy, or in the prone position in cases of bilateral reconstruction.
Flap dissection was performed in all cases that required the use of electrocautery, using all the available muscular venter. After identifying the point of insertion of the LDMF and confirming the location of the vascular pedicle (previously delineated), the dissection was interrupted and the flap was transposed to the new breast site. The closure of the dorsum was primarily made using adhesion sutures of 2.0 Vicryl applied between the detached flaps and the thoracic wall. A vacuum drain was used in all patients in the dorsal area and in the area of the new breast for an average of 8 to 10 days. The skin was sutured with subdermal and intradermal 4.0 Monocryl sutures.
After suturing and dressing of the dorsal area, the patients were placed in the prone position and antisepsis was performed on the new breast using a prosthesis or permanent expander, according to the indication in each case. The LDMF was fixed to the thoracic wall around the prosthesis in most of the cases by suturing between the latissimus dorsi muscle and the anterior thoracic wall structures under the implant.
In 8 of the 21 reconstructions, the implant placement and coverage were performed using novel techniques. The pectoralis major muscle was dissected, and its inferomedial portions were released from the rib cage and the sternal edge for placement of a silicone prosthesis or definitive expander (Figure 3). The LDMF was sutured with 2.0 Vicryl over the pectoralis major muscle, ensuring greater thickness for coverage in the upper and medial quadrants.
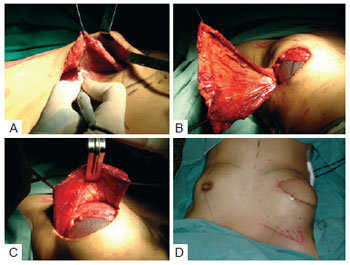
Figure 3 - Strategy used in this study for coverage of the implant using a double muscle layer. In A, dissection of the subpectoral cavity. In B, placement of the subpectoral implant. In C, fixation of the latissimus dorsi muscle flap over the pectoralis major muscle and implant. In D, good contour of the upper and medial quadrants.
In 18 reconstructions, high profile round silicone prostheses were used (9 of polyurethane and 9 textured) and Becker-50 expanders were used in 3 reconstructions. The choice of the type of prosthesis (polyurethane or textured) was random.
The subjective analysis of the results of reconstructive surgery was achieved by means of a questionnaire distributed to the patients (Figure 4).

Figure 4 - Questionnaire for the subjective assessment of breast reconstruction results with the latissimus dorsi muscle and prosthesis or expander.
RESULTS
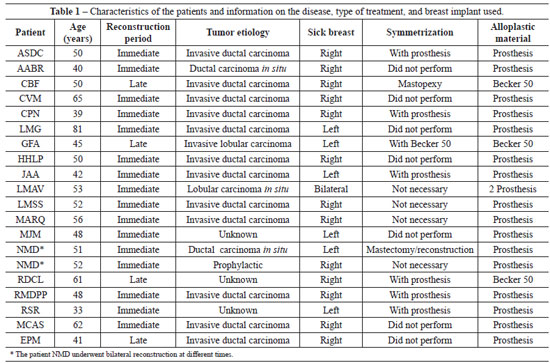
Between June 2006 and June 2009, 19 patients underwent breast reconstruction and 2 of them underwent bilateral surgery, totaling 21 reconstructions with an average follow-up period of 14.23 months. The characteristics of the patients and procedures performed are shown in Table 1.
One of the patients had bilateral disease and underwent immediate bilateral reconstruction. Another patient underwent reconstruction with LDMF and a bilateral prosthesis. Both reconstruction surgeries were performed at different times - the first surgery due to cancer and the second after prophylactic adenomastectomy.
The hospitalization period was 1 and 2 days in 14 and 5 patients, respectively. Four patients had a seroma in the dorsum, which was resolved with aspiration during the initial examination. One patient who underwent late reconstruction had a history of previous radiotherapy, and presented with flap dehiscence in the area of irradiation. The patient developed local cellulitis, which resolved after 2 weeks of anti-microbial treatment. Another patient had a reaction to the suture threads used in the dorsum and in the new breast (Figure 5).
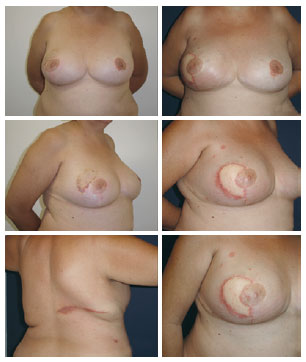
Figure 5 - Latissimus dorsi muscle flap and prosthesis under a double muscle layer: foreign body reactions are observed in all scars.
There were no significant complications associated with the flaps or relevant systemic complications. In one case of late reconstruction with LDMF and Becker-50 expander, it was necessary to perform a new procedure to reposition the implant, which was low in relation to the contralateral breast.
After the staging (TNM) of the patients that had undergone immediate reconstructions, 14 cases were ranked as T1 or T2 and only 2 cases as T3. None of the patients was diagnosed with metastatic disease. Sentinel lymph node analysis was positive in 2 patients, who were promptly subjected to axillary dissection; in a third patient, a second procedure was necessary for axillary dissection after the detection of positive lymph nodes in the histopathology results that were not previously identified.
Of the 15 patients undergoing immediate reconstruction, only one had a previous indication for radiotherapy; however, after the postoperative staging, another 5 patients were referred for radiotherapy. The patient with a known radiotherapy indication preoperatively did not want to postpone the reconstruction, and did not agree to the TRAM procedure. In this patient, reconstruction with an expander was not possible because of the amount of skin that would have to be resected as both tumors were too close to the skin. Of the 4 patients who underwent late reconstructions, 3 had already been treated with radiotherapy postoperatively and showed the typical sequelae of an irradiated thorax.
Of the 5 patients who underwent radiotherapy in the postoperative period after immediate reconstructions, 4 had capsular contracture (2 with Baker II and 2 with Baker III). Only 1 patient with Baker III requested correction of the capsular contracture, which was performed with a capsulotomy and replacement of the prosthesis by another one of greater volume for better symmetry.
Patients in whom the prosthesis or expander was placed under the muscle layer (sub-group of 7 patients and 8 reconstructions) showed less noticeable creases in the upper and medial quadrants, and the rims of the implants were well concealed, resulting in a more natural appearance compared with reconstructions in which the implant was only covered with the LDMF (Figure 6).
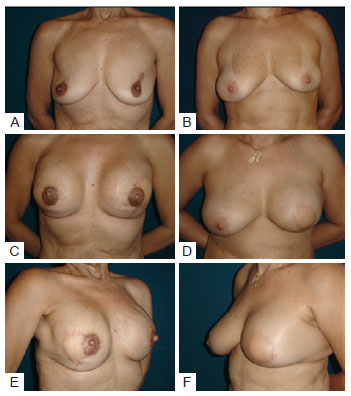
Figure 6 - In A, C, and E, patient that underwent bilateral reconstruction. Creases are present in the upper quadrants. In B, D, and F, reconstruction where placement of the prosthesis under a double muscle layer resulting in coverage of the implant and absence of creases.
Of 19 patients, 8 underwent symmetrization and 6 had reconstruction of the NAC. The symmetrization was performed with similar prostheses to those used in the reconstruction in 6 cases, including an expander in 1 case and only mastopexy in 1 case. NAC reconstructions were performed with skate-type local flaps and dermal-pigmentation (Figure 7)13.
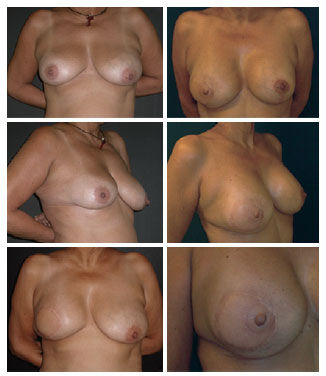
Figure 7 - A 50-year-old patient who underwent mastectomy and right breast reconstruction with the latissimus dorsi muscle flap and prosthesis. The symmetrization was performed with mastopexy and prosthesis, and the reconstructed nipple-areola complex has not yet undergone dermal-pigmentation.
The patients' responses to the questionnaire demonstrated a high degree of satisfaction, with a low incidence of functional alterations (Figure 8).
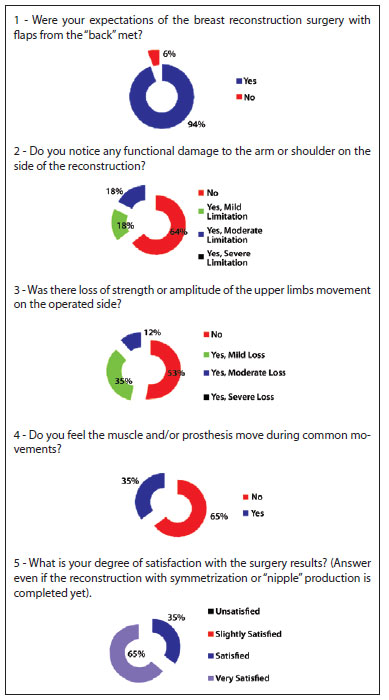
Figure 8 - Analysis of the responses to the 5 questions in the questionnaire.
DISCUSSION
The benefits of immediate breast reconstruction after tumor removal are unquestionable nowadays. Breast reconstruction does not alter the biological behavior of the cancer and it does not affect the treatment. Studies such as those of Bostwick et al.10 and Dinner & Peters14 describe the integration of plastic surgery into the management of breast cancer as a crucial event. The work of the plastic surgeon is essential for the recovery of a patient's self-esteem as it increases volume and improves the shape and natural appearance of the thorax, which would otherwise be a permanent cause of stigma and marked by a mastectomy scar.
The era between the first breast reconstructions performed by Bostwick et al.10 and the currently performed surgeries with LDMF has been marked by the development of improved silicone prostheses and expanders, which have expanded the surgical possibilities for the repair of different defects.
The early diagnosis of breast cancer has caused an increase in the number of breast reconstructions with local flaps, with expanders, and with LDMF much to the detriment of the TRAM, which has greater local and systemic morbidity15. Thus, the LDMF associated with a prosthesis or expander is an excellent option for breast reconstruction after skin-saving mastectomies in patients in the early stages of breast cancer.
Patients that are referred for immediate or late breast reconstruction often have absolute or relative contraindications to reconstructions using TRAM. However, the use of LDMF without prostheses in these cases may be unsuccessful and sometimes frustrating owing to the occurrence of muscle atrophy and the consequent loss of volume and shape.
Although there is consensus with regard to the use of breast reconstruction techniques with autologous tissues in patients with known or suspected need for postoperative radiotherapy, there are numerous situations in which the use of implants with or without flaps is indicated 16.
In the present study, the Becker 50 permanent expander was used in 2 patients who underwent late reconstruction after radiotherapy and whose skin island of the LDMF was not sufficient to cover the prosthesis and enable adequate breast reconstruction. These patients were not candidates for TRAM because of prior abdominoplasty in 1 patient and lack of sufficient tissue in the infraumbilical region in the other one. In a third patient, the Becker 50 expander was used to recover the medial pole of a new breast reconstructed with TRAM that had liponecrosis and radiodermatitis.
Only 1 patient in the present study fulfilled the criteria for postoperative radiotherapy. As it was a very young patient with a non-donor abdomen who required immediate reconstruction and with tumors very close to the skin, which would also have to be removed, the surgical indication was LDMF with prosthesis. Within a period of less than 1 year after the radiotherapy, this patient had a Baker III capsular contracture and underwent surgery for capsulotomy, expansion of the cavity, and replacement of the 345 ml polyurethane implant with a 485 ml implant.
According to the current published literature, there are no contraindications to immediate reconstruction, and opposing this surgery would be contrary to most of the patients' beliefs that it is always possible to have an immediate reconstruction. Therefore, by performing immediate reconstruction, the surgeon often assumes a high risk of capsular contracture, which can reach an incidence rate of 68% according to data from McCarthy et al.17. However, this same study reported that the rate of capsular contracture in breast reconstructions with alloplastic materials, even without postoperative radiotherapy, is 40%. Final success rates of reconstructions were 90% with radiotherapy and 99% without radiotherapy.
The latissimus dorsi muscle is approximately 16.3-cm wide and 29.2-cm long, and the fatty layer underneath Scarpa's fascia can be mobilized together with the muscle to improve the coverage of the implants or even to add volume18. In thin and/or sedentary patients, the latissimus dorsi muscle is limited in size, and an extra layer of coverage can be added by using the pectoralis muscle to create a pouch for the implant without complications.
This technique was first used in a patient who, after agreeing to have an immediate reconstruction with a permanent expander or LDMF and prosthesis, was found to be ineligible for the expander. After dissection of the cavity for the supposed insertion of the definitive expander, the adipose pad under the pectoralis muscle was found to be inadequate for reconstruction because of the limited subcutaneous tissue present. In addition, dissection of the LDMF revealed that it was too thin to be used in isolation and the use of the LDMF in combination with the latissimus dorsi was necessary to cover the prosthesis. The result of this reconstruction was satisfactory with respect to the coverage of the implant, the shape of the new breast, and the absence of palpable creases in the upper quadrants of the breast (Figure 9). This procedure is used when the skin-saving mastectomy leaves a thin flap. Another advantage of this strategy is that the implant is enclosed in the subpectoral cavity and is placed in a good position in relation to the inframammary crease of the contralateral breast, thus reducing the risk of migration.
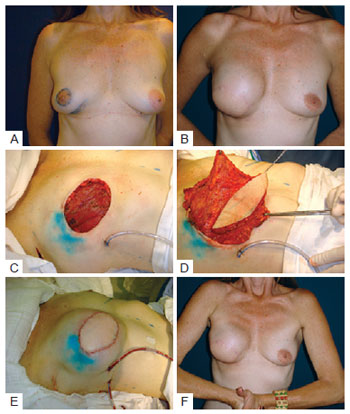
Figure 9 - In A, patient with ductal carcinoma in situ, multifocal, showing both biopsied areas that were resected en bloc. In B, late result after reconstruction with the latissimus dorsi muscle flap. In C, mastectomy with wide skin resection. In D, flap transposition. In E, immediate postoperative period. F, prosthesis under the pectoralis major and flap of the latissimus dorsi muscle.
In immediate reconstructions associated with considerable mammary ptosis, the skin envelope is readjusted over the volume generated, with decortication of the epidermis and positioning of the breasts according to the requirements of each case (Figure 10).
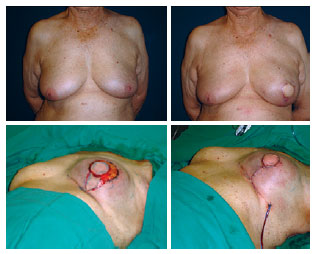
Figure 10 - Reconstruction of the left breast with the latissimus dorsi muscle flap and prosthesis: resection of excess skin and inverted T closure.
The use of implants positioned underneath the pectoralis muscle without concealing the upper poles of the implant under a double layer of muscle has been reported in the literature19. These surgeries are based on the use of the pectoralis major muscle in addition to the LDMF "in pouch," and the main concern is to provide additional protection to larger implants to prevent skin necrosis and further complications inherent to skin-saving mastectomies. With this purpose, the latissimus dorsi muscle is sutured at the lower edge to achieve a complete closure of the prostheses at the lower pole.
In the present study, the 8 cases in which a double layer of coverage was used for the implant showed a significant improvement in the aesthetic results according to the assessment of the patients and the surgeon and in comparison with surgeries performed by placing the implant over the pectoralis major muscle.
CONCLUSION
Plastic surgery plays an important role in the treatment of patients with breast cancer.
Breast reconstruction with LDMF is widely applicable and can correct almost all post-mastectomy defects. A high degree of satisfaction among the patients was achieved and the results were acceptable, with few functional repercussions.
The use of a double muscle layer to provide better coverage of the implant and to create an adequate cavity significantly improved the aesthetic results with regard to the upper quadrants of the reconstructed breasts. It is an innovative procedure that should be added to the current techniques of breast reconstruction with LDMF and implants.
REFERENCES
1. Halsted WS. I. The result of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June, 1889 to January, 1894. Ann Surg. 1984;20(5):497-555.
2. Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337(14):956-62.
3. Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999; 353(9165):1641-8.
4. Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20-69 years. Lancet. 2000; 355(9217):1822.
5. Petit JY, Lê MG, Mouriesse H, Rietjens M, Gill P, Contesso G, et al. Can breast reconstruction with gel-filled silicone implants increase the risk of death and second primary cancer in patients treated by mastectomy for breast cancer? Plast Reconstr Surg. 1994;94(1):115-9.
6. Drever JM. Total breast reconstruction with either of two abdominal flaps. Plast Reconstr Surg. 1977;59(2):185-90.
7. Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69(2):216-25.
8. Gandolfo EA. Breast reconstruction with a lower abdominal myocutaneous flap. Br J Plast Surg. 1982;35(4):452-7.
9. Tansini I. Sopra il mio nuovo processor di amputazione della mammella. (coverage of the anterior chest wall following mastectomy). Guz Mal Ital. 1906;57:141.
10. Bostwick J 3rd, Vasconez LO, Jurkiewicz MJ. Breast reconstruction after a radical mastectomy. Plast Reconstr Surg. 1978;61(5):682-93.
11. Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109(7):2265-74.
12. de la Torre JI, Fix RJ, Gardner PM, Vasconez LO. Reconstruction with the latissimus dorsi flap after skin-sparing mastectomy. Ann Plast Surg. 2001;46(3):229-33.
13. Shestak KC, Nguyen TD. The double opposing periareola flap: a novel concept for nipple-areola reconstruction. Plast Reconstr Surg. 2007; 119(2):473-80.
14. Dinner MI, Peters CR. Breast reconstruction following mastectomy. Surg Clin North Am. 1978;58(4):851-68.
15. Cammarota MC. Reconstrução de mama com retalho de grande dorsal: estudo das pacientes operadas no período de junho de 2003 a junho de 2005 [tese para concurso de professor titular]. In: XLIII Congresso Brasileiro de Cirurgia Plástica; 2006 Nov 11-14; Recife, Brasil.
16. Zimmerman RP, Mark RJ, Kim AI, Walton T, Sayah D, Juillard GF, et al. Radiation tolerance of transverse rectus abdominis myocutaneous-free flaps used in immediate breast reconstruction. Am J Clin Oncol. 1998;21(4):381-5.
17. McCarthy CM, Pusic AL, Disa JJ, McCormick BL, Montgomery LL, Cordeiro PG. Unilateral postoperative chest wall radiotherapy in bilateral tissue expander/implant reconstruction patients: a prospective outcomes analysis. Plast Reconstr Surg. 2005;116(6):1642-7.
18. Hazan ASB, Nahas FX, Barbosa MVJ, Piñeda E, Juliano Y, Ferreira LM. Análise anátomo-histológica das subunidades musculares do músculo grande dorsal. Rev Soc Bras Cir Plást. 2006;21(4):203-10.
19. Munhoz AM, Montag E, Fels KW, Arruda EG, Sturtz GP, Aldrighi C, et al. Outcome analysis of breast-conservation surgery and immediate latissimus dorsi flap reconstruction in patients with T1 to T2 breast cancer. Plast Reconstr Surg. 2005;116(3):741-52.
1. Member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery) - SBCP, plastic surgeon at the Plastic Surgery Department of Hospital Daher Lago Sul, Brasília, DF, Brazil.
2. Associate member of SBCP, plastic surgeon, Brasília, DF, Brazil.
3. Member of SBCP, head of the Plastic Surgery Department of Hospital Daher Lago Sul, Brasília, DF, Brazil.
4. Resident physician of the Plastic Surgery Department of Hospital Daher Lago Sul, Brasília, DF, Brazil.
Jefferson Di Lamartine
SCN Quadra 2 - Torre A - Salas 1121/1123 - 11º andar - Shopping Liberty Mal
Brasília, DF, Brazil - CEP 70712-903
E-mail: jdilamartine@dilamartine.com.br
Manuscript submitted to the SGP (Publications Management System) of RBCP.
Article received: September 21, 2011
Article accepted: January 31, 2012
Study conducted at Instituto de Cirurgia Plástica Di Lamartine, Brasília, DF, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter