

Original Article - Year 2012 - Volume 27 -
Predictive factors of good aesthetic results in conservative surgery for breast cancer
Fatores preditivos para um bom resultado estético em cirurgias conservadoras por câncer de mama
ABSTRACT
BACKGROUND: The aim of conservative surgery for treatment of breast cancer is obtaining satisfactory aesthetic results. Segment-based surgery, which must be followed by radiotherapy (RT), is considered the treatment of choice for most breast cancer patients. Single-dose intraoperative radiation in the tumor bed (IORT) is a promising radiation technique that is more rapid than conventional RT and less exhausting for the patient. The aim of this study was to evaluate the final aesthetic results of patients who had undergone conservative surgery and treatment for early-stage breast cancer consisting of primary closure of the operative wound and use of adjuvant radiotherapy, either conventional RT or IORT, and assess the impact of several variables on the results.
METHODS: Primary closure of the operative wound after conservative breast cancer treatment was performed in 66 patients. The patients were evaluated and photographed and their data collected from medical records.
RESULTS: Some degree of asymmetry was observed in 40.4% of patients. The surgically treated breast frequently appeared more aesthetically pleasing than the healthy breast, especially in patients with large breasts (P = 0.052), in whom resection of the upper quadrants improved the degree of ptosis and thus improved the appearance of the treated breast (P = 0.002 and P= 0.001, respectively). Use of periareolar incision (P = 0.008) was found to be a predictor of good aesthetic results while the comorbidity of diabetes mellitus and the use of chemotherapy were found to be predictors of poor results (P = 0.046 and P = 0.073, respectively).
CONCLUSIONS: Some degree of asymmetry often results in patients for whom remodeling of mammary tissue is not possible. The factors of use of periareolar incision, large breast volume, and tumor location in one of the upper quadrants are predictors of a good aesthetic outcome, while use of chemotherapy, diabetes mellitus, and tumor location in the lower quadrants are negative predictors. Use of IORT yields aesthetic outcomes comparable to those of conventional RT in terms of extent of scarring.
Keywords: Breast neoplasms. Radiotherapy. Plastic surgery/methods.
RESUMO
INTRODUÇÃO: O câncer de mama é uma doença frequente e a cirurgia segmentar associada à radioterapia é considerada o tratamento de escolha para a maioria das pacientes. O objetivo da cirurgia conservadora é a obtenção de resultado estético satisfatório. O tratamento irradiante é imperativo e a radioterapia intraoperatória é uma promissora técnica de irradiação. O objetivo deste estudo foi avaliar o resultado estético final de pacientes com câncer de mama em estádio inicial submetidas a cirurgia conservadora, fechamento primário da ferida operatória e radioterapia adjuvante (radioterapia convencional ou radiação intraoperatória em dose única no leito tumoral), bem como analisar a influência de outras variáveis no resultado cosmético.
MÉTODO: Fechamento primário da ferida operatória após tratamento conservador do câncer de mama foi realizado em 66 pacientes. As pacientes foram avaliadas e fotografadas e seus dados foram coletados dos prontuários médicos.
RESULTADOS: Observou-se que 40,4% das pacientes apresentaram algum grau de assimetria; contudo, frequentemente a mama operada se apresentava esteticamente melhor que a mama sadia, em especial em mamas grandes (P = 0,052) e ressecções dos quadrantes superiores, que melhoravam o grau de ptose e ficavam, consequentemente, mais bonitas. (P = 0,002 e P = 0,001, respectivamente). A incisão periareolar (P = 0,008) é fator preditivo de bom resultado estético; em contrapartida, o diabetes e a quimioterapia predizem mau resultado (P = 0,046 e P = 0,073, respectivamente).
CONCLUSÕES: Quando não é possível remodelar o tecido mamário, a assimetria é frequente. Incisões periareolares, mamas volumosas e tumor em quadrante superior são considerados fatores preditivos de bom resultado estético, e como valor preditivo negativo, diabetes melito, quimioterapia e tumor em quadrante inferior. A radiação intraoperatória em dose única no leito tumoral proporciona tratamento irradiante mais rápido e menos desgastante para a paciente e demonstrou equivalência no aspecto estético sobre a cicatriz e a mama.
Palavras-chave: Neoplasias da mama. Radioterapia. Cirurgia plástica/métodos.
Breast cancer is the second most common type of cancer in the world. Apart from non-melanoma skin cancer, it is the most common cancer in women, being responsible for approximately 22% of all cancer cases newly diagnosed every year in women. Breast cancer is second most common cause of death by cancer in women, preceded only by lung cancer1,2, with a worldwide survival rate after 5 years of 61%1. In 2011, it had been estimated that 230,480 women and 2,140 men would be diagnosed with invasive breast cancer in the United States and that 39,970 (39,520 female and 450 male) deaths would be attributable to this diagnosis2. In Brazil, 49,240 new cases had been expected in 2010, indicating a breast cancer incidence of 49 cases per 100,000 individuals. In the Southeast region, the incidence is even higher, estimated at 65 per 1000,000 inhabitants. In 2010, it was estimated that 11,860 (11,735 female and 125 male)1 deaths would be attributable to breast cancer.
Risk factors for breast cancer in women are closely associated with reproductive factors, including early menarche, use of oral contraceptives, use of hormone replacement therapy (HRT), nulliparity, first pregnancy after age 30, and late menopause. As such, it is associated with the urbanization of society, and thus women of high socioeconomic status are particularly at risk1. Although breast cancer is relatively rare before 35 years of age, its incidence increases rapidly and progressively thereafter. According to the World Health Organization (WHO), the cancer population-based cancer registries of several continents1 indicated a 10-fold increase in incidence rate, adjusted by age, in the 1960s and 1970s. In developed countries, incidence rates for breast cancer started to decline in 2000 and have been generally stable since 2003. At the same, death rates have been consistently decreasing since 1990, primarily due to early detection, improved treatment, and, most recently, decrease in incidence2. In Brazil, cancer mortality rates remain high, most likely due to diagnosis at advanced stages1.
Although the prognosis remains less favorable in Brazil and other developing countries, it is important to recognize that in the past several decades have seen enormous advances in multidisciplinary breast cancer treatment throughout the world. Several factors have contributed to ensuring the provision of less aggressive but increasingly effective and safe forms of detection and treatment that value quality of life and have favorable aesthetic results. These include early diagnosis, determination of stage through axillary sentinel lymph node (LS) biopsy, radiotherapy (RT), chemotherapy therapy (CT), hormone therapy (HT), and conservative surgical breast remodeling3.
For over 80 years, radical surgical treatment of breast cancer had been the universally accepted surgical approach. Veronesi et al.'s4-6 research provided for significant advances in surgical treatment by showing that in selected cases (generally, cases at initial stages), conservative surgical treatment led to outcomes similar to those obtained with radical surgery concerning locoregional relapse and global survival7,8. With this finding, conservative breast surgery has become increasingly common over the past 30 years, being accepted worldwide as the treatment of choice in up to 80% of primary breast cancer cases8. The advantages of using conservative surgical techniques are reduction in breast deformity by preservation of the majority of the mammary parenchyma, reduction in morbidity, reduction of surgical impact on breast functioning, and improved aesthetics, all of which improve the psychosocial aspects associated with tumor resectioning3,4,7,8.
Although most patients initially expressed satisfaction with the possibility of maintaining their breast after tumor resection, it has become clear that expectations have been increasing, with many patients now believing that conservative surgery should result in healthy-looking, symmetrical breasts without residual deformity. However, these results are not always obtained in practice, with the aesthetic results being considered unacceptable in up to 30% of the cases8. Considering that an acceptable aesthetic result is the major aim of conservative surgery when compared to mastectomy9, conservative surgery generally does not fulfill expectations. It particularly fails to do so when resected mammary tissue is repaired by simply pulling together the margins of the surgical area, a technique that may lead to difficulty with repairing sequels and to aesthetic deformities consequent to deficiency of skin, subcutaneous cellular, and gland tissue; loss of projection; and retraction and distortion of the breast and papillary-areolar complex (CAP). These defects may be worsened by external postoperative RT, which must be performed almost daily for 4 to 6 weeks following surgery7-11. Besides increasing risk of local complications due to the presence of an operative wound, RT also poses the risk of vascular changes, intense fibrosis, aesthetic dissatisfaction, and physical and psychological discomfort12-17.
Veronesi et al.12 found that over the course of breast cancer treatment, the majority of cases of relapse occurred in regions adjoining the site of previous segment resectioning, and only a minority in other mammary quadrants. This finding suggests that in cases of conservative breast surgery, partial mammary irradiation yields results comparable to those of external total irradiation and provides several benefits, including a briefer treatment period, lower costs, fewer side effects, and possibly better aesthetic results. Among the several types of partial breast radiation aiming at minimizing total treatment time while maintaining historical levels of local relapse, single-dose intraoperative radiation in the tumor bed (IORT) with electrons has been found to be particularly promising, with phase I and II studies confirming its safety and effectiveness15-20. In Brazil, a few centers already use this new and promising radiation technique.
Changes in the traditional conceptual approach to breast cancer treatment, which is based on the use of invasive oncological procedures, requires an adaptation to the reparation concept10. Immediate corrections are now popular because they yield favorable aesthetic and psychosocial results without significantly interfering with the diagnosis or increase risk of relapse. Depending on the residual volume of the contralateral breast, patient preference, local tissue conditions, and often (erroneously) on the patient healthcare plan, the margins of the surgical area may be simply pulled and stitched together or the remaining mammary tissue may be remodeled. In general, the breast remodeling techniques performed to recover lost volume by inserting an implant or performing more elaborate techniques using myocutaneous grafts are mostly based on mammoplasty and mastopexy, with the goal of symmetrization of the contralateral side. Being considered efficient, these techniques are widely used and increasingly referred to as forms of "oncoplastic surgery"21,22.
Considering that the efficacy and healing potential of current cancer treatments are already established, it is now important to evaluate the different aesthetic results that they yield. Dong so will assist surgeons in selecting among the different breast restructuring and RT approaches to use with their patients. In the many studies23-25 that have attempted to identify the predictors of good aesthetic results, the most commonly evaluated factors have been scar visibility and appearance, patient age, body mass index (BMI), tumor localization and size compared to breast size, and use of adjuvant therapies such as RT and CT8,23-26. However, the most widely used evaluation methods are invariably subjective, leading to results of questionable reliability and, therefore, doubts regarding the extent to which these factors truly affect cosmetic results24,27. Numerous studies have addressed the topic of early-stage breast cancer treatment3-8,11-14,21,28, but few have evaluated aesthetic outcomes9,10,22-27,29-43 and even fewer the impact of IORT on outcomes15-20. To fill this research gap, this study evaluated the impact of several variables on the final treatment and aesthetic outcomes of patients with early-stage breast cancer treated with conservative surgery for tumor resection and primary wound closure followed by adjuvant RT, either conventional RT or IORT.
METHODS
This study prospectively evaluated the progression of 66 breast cancer patients with invasive ductal carcinoma defined as early stage, i.e., a cancer stage earlier than T2N1M0, treated using a conservative surgical approach that aimed at primary closure of the surgical margins either with or without mammary prosthesis and no remodeling of the mammary tissue. All surgeries were performed between May 2008 and January 2011, and the postoperative follow-up period ranged from 6 to 20 months. All patients signed an informed consent form after verbally agreeing to participate in the study. The project was submitted to the Ethics and Research Committee of the A. C. Camargo Hospital and approved on October 14, 2008 under number 1122/08. Figure 1 shows the most common breast cancer treatments currently provided, with the patients eligible for this study highlighted in blue.
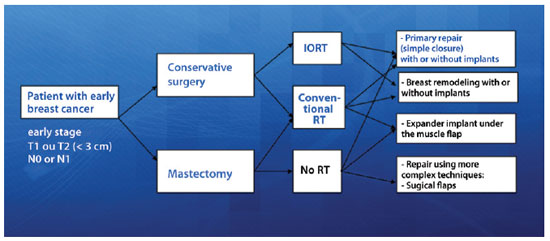
Figure 1 - Types of treatment for patients with early-stage breast cancer. In blue, In blue is the group of patients eligible for this study. IORT = single-dose intraoperative radiation in the tumor bed; RT = radiation therapy.
Inclusion criteria
The following were the inclusion criteria for this study:
Sex: Female. Type and stage of tumor: -Breast cancer type: Ductal invasive adenocarcinoma stage I (T1N0M0) or stage II (T0N1M0, T1N1M0, T2N0M0, or T2N1M0, with T2 < 3 cm, with N1< 3 involving lymph nodes);
-Negative surgical margins.Surgery for tumor resection: Sectorectomy, tumorectomy, quadrantectomy, or lumpectomy, also referred to as segment-based surgery; Associated therapy: Either conventional RT or IORT; Breast restructuring: Primary closure of the operative wound by the pulling together of all anatomical planes or immediately reconstruction with silicone gel-filled breast implants without repair or aesthetic surgery of the contralateral breast.
Exclusion criteria
The following were the exclusion criteria for this study:
Sex: Male. Previous history of any of the following: -RT in the breast or thorax for any reason;
-Collagen disease;
-Psychiatric illness or any other condition that might prevent study compliance.Type and stage of tumor disease -Positive margins upon anatomopathological evaluation;
-Type of tumor other than invasive adenocarcinoma;
-Any tumor lesion in stage III or IV;
-Stage II with diameter 3 cm or larger;
-Presence of 3 or more positive lymph nodes;
-Positive lymph nodes other than axillary nodes;
-Multicentric carcinoma in more than 1 quadrant or carcinomas that are separated by 4 cm or more;
-Previous history of breast cancer in the same breast.Associate therapy: Any mastectomy technique, whether simple or skin- sparing mastectomy; a modification of the Patey, Madden, and Auchincloss radical mastectomy; or any other form of radical mastectomy. Breast restructuring: -Reconstruction that occurred relatively late;
-Remodeling after tumor removal;
-Reconstruction with a pedunculated or free myocutaneous flap;
-Use of expanders before silicone implant.
In the postoperative period, patients were re-evaluated and photographed from the front while resting and while elevating the upper limbs, as well as from the left and right oblique positions and from the left and right sides. A close-up photograph of the scar was also taken (Figure 2). Data regarding patient physical and clinical variables, including breast characteristics, co-morbidities, use of adjuvant therapies, quadrant treated, and type of incision, were collected by retrospective review of medical records. Degree of breast ptosis was determined using the Regnault classification with some modification according to the following scheme (Figure 3):
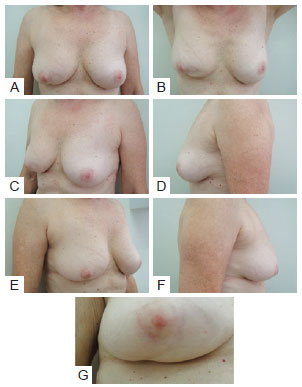
Figure 2 - In A, Patient photographed from the front while resting. In B, Patient photographed from the front while elevating upper limbs. In C, Patient photographed from a left oblique position. In D, Patient photographed from the left side. In E, Patient photographed from a right oblique position. In F, Patient photographed from the right side. In G, Close-up photograph of the scar.
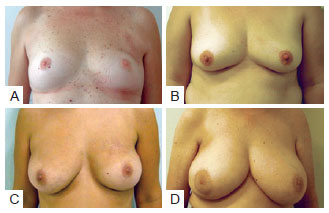
Figure 3 - In A, Patient without ptosis and estimated breast volume less than 200 cc. In B, Patient with slight ptosis and estimated breast volume of 200 cc to 400 cc. In C, Patient with moderate ptosis and estimated breast volume of 400 cc to 600 cc. In D, Patient with pronounced ptosis and estimated breast volume of higher than 600 cc.
Score 0 or no ptosis: Absence of submammary grooves; Score 1 or slight ptosis (partial ptosis or pseudoptosis in the original classification): Presence of submammary groove below the areola; Score 2 or moderate ptosis (degree I in the original classification): Presence of submammary groove with areola within it; Score 3 or pronounced ptosis (degrees II and III in the original classification): Presence of submammary groove above the areola.
Evaluation of aesthetic results was performed using the Moro and Ciambellotti27 classification according to the following scheme (Figure 4):
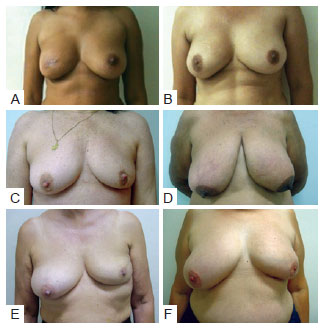
Figure 4 - Examples of assessment for the Moro & Ciambellotti classification. In A and B, patients with aesthetic results considered excellent. In C and D, patients with aesthetic results considered satisfactory. In E and F, patients with aesthetic results considered poor.
Excellent: Absence of CAP asymmetry, loss of mammary volume, or mammary retraction; Satisfactory: Presence of CAP asymmetry, more than 1/3 loss of mammary volume, or mammary retraction; Poor: Presence of 2 or more of the factors described above.
Extent of scarring, changes in shape and position, dynamic and static retraction, and shape and coloration of the CAP were evaluated. The evaluation concluded with an assessment of the approximate volume of the healthy breast and determination of whether the healthy or treated breast had a more pleasing appearance, disregarding the appearance of the scar and considering only volume changes, shape, and CAP position, conducted by 3 plastic surgeons. The factors evaluated were classified into the following categories in accordance with the literature9,24.
Patient-dependent factors: Age, BMI, race/ethnicity, co-morbidities (e.g., diabetes mellitus [DM], systemic arterial hypertension, and/or tobacco use), and breast size; Tumor-dependent factors: Tumor size and localization; Treatment-dependent factors: Type of incision, size of resected piece, and adjuvant treatments used (i.e., RT, CT and/or HT).
Exploratory analysis of the data was conducted and associations between the variables were verified using the exact Fisher test at a 95% (P < 0.05) confidence level and XLSTAT software.
RESULTS
Surgical treatment
Among the 66 patients identified as having undergone primary closure of the operative wound after conservative treatment of breast cancer, all were found to have also undergone RT. Regarding the type of RT, 19 patients had undergone IORT at a total dose of 21 Gy while 47 had undergone conventional RT at a total dose of 55 Gy, with the most widely used approach of the latter being 25 sessions at a dose of 180cGy of the entire targeted breast and 5 reinforcement sessions at a dose of 200 cGy in the tumor bed.
Patient-dependent factors
The mean age of the patients was 55.9 years and ranged from 33 to 82 years.
Regarding race/ethnicity, 56 identified as white, 4 as Asian, 4 as biracial, and 2 as black.
Regarding weight classification according to BMI, 1 patient was underweight (BMI < 18.4), 29 patients were normal (BMI < 24), 21 were overweight, and 15 were obese. Regarding comorbidities, 26 patients were hypertensive, 9 had DM, 4 were smokers, and 11 were ex-smokers. The relationship between extent of scarring and comorbidities is shown in Figure 5.
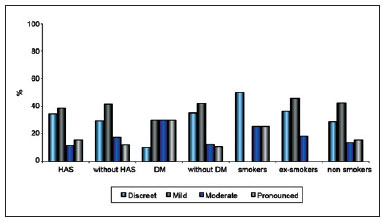
Figure 5 - Relationship between extent of scarring and patient comorbidities.
Tumor-dependent factors
Regarding tumor localization, the tumor was located in the upper lateral quadrant (QSL) in 22 patients, the upper quadrant (UQS) in 10, the intersection of the lateral quadrants (UQL) in 9, the medial upper quadrant (QSM) in 8, the medial lower quadrant (QIM) in 5, the central quadrant (QC) in 4, the intersection of the lower quadrants (UQI) in 4, the lower lateral quadrant (QIL) in 3, and the intersection of the medial quadrants (UQM) in 1.
Treatment-dependent factors
Regarding type of incision used in tumor resectioning, 30 patients had undergone arched incision; 16 periareolar incision; 14 radial incision; 6 lower vertical incision, either in the groove or in the "T" (Figure 6). The relationship between extent of scarring and type of incision or procedure is shown in Figure 7.
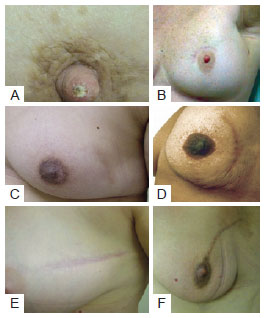
Figure 6 - Different type of incision used in tumor resectioning. In A and B, patients with periareolar incision. In C and D, patients with arched incision. In E e F, patients with radial incision.
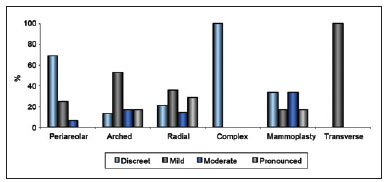
Figure 7 - Relationship between extent of scarring and type of incision or procedure.
The relationship between extent of scarring and type of RT is shown in Figure 8.
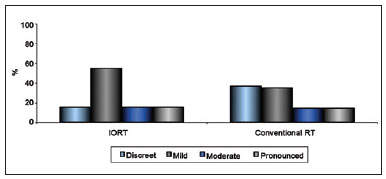
Figure 8 - Relationship between extent of scarring and type of radiotherapy.
Among all 66 patients, only 7 had undergone implantation of mammary implants to compensate for significant volume deficit, and only 31 had required and consequently undergone adjuvant CT. In contrast, 62 patients had required HT, such as tamoxifen, arimidex, or imatinib therapy.
Evaluation
Regarding scar quality, the extent of scarring was considered subtle and almost absent in 21 patients, slight in 26, moderate in 10, and pronounced in 9. Regarding change in CAP position, no change was observed in 18 patients; slight change in 40, of which the change was positive in 32, negative in 5, and neither positive nor negative in 3; moderate and positive change in 4; and not possible to evaluate in 4 because the areola had been resected together with the tumor. Regarding categorization using the Moro and Ciambellotti classification27, the result was considered excellent for 18 patients, satisfactory for 22, and poor for 26. Breast volume was estimated at less than 200 cc in 6 patients, 200 to 400 cc in 12, 400 to 600 cc in 33, and greater than 600 cc in 15. The relationship between the extent of scarring and estimated volume of the breast is shown in Figure 9.
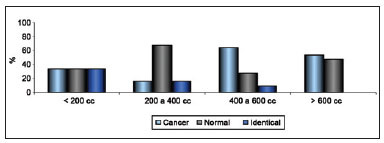
Figure 9 - Relationship between extent of scarring and estimated breast volume.
Regarding the degree of mammary ptosis, none was observed in 4 patients, mild ptosis in 10, moderate ptosis in 28, and pronounced ptosis in 24. Regarding aesthetic quality, the surgically treated breast and the untreated breast were considered equally aesthetically pleasing in 7 patients, the untreated breast more aesthetically pleasing in 26, and the treated breast more aesthetically pleasing in 33. Regarding changes in shape, none was observed in 24 patients, minor changes in 27, moderate changes in 12, and pronounced changes in 3, with all changes considered an indication of worsening shape. Regarding extent of static retraction, none was observed in 36 patients, slight retraction in 24, moderate in 5, and pronounced in 1. Regarding extent of dynamic retraction upon lifting the upper limbs, none was observed in 30 patients, slight retraction in 17, moderate in 14, and pronounced in 5 (Figure 10).
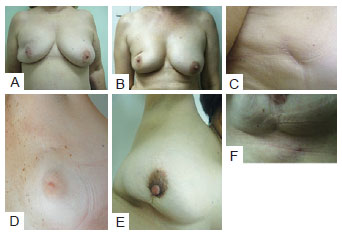
Figure 10 - In A, B and C: Examples of static retractions. In D, E and F, examples of dynamic retraction.
DISCUSSION
Many studies have evaluated the aesthetic results of conservative breast surgery and identified the factors that might influence them. Regarding patient-dependent factors, being relatively thin, young, and light complexioned is associated with favorable aesthetic results. Regarding treatment-dependent factors, resectioning of a large quantity of tissue and use of CT have been associated with unfavorable aesthetic results9,24. In their evaluation, some studies have assumed the existence of a direct relationship between scar visibility and extent of change in body image, and many have confirmed the existence of a relationship between scar visibility and aesthetic appearance24,25. As the results of these studies indicate that the extent of surgical scarring influences final aesthetic appearance, this study evaluated several factors that might directly impact the extent of scarring. Of the patient-dependent variables that were evaluated, which included age, BMI, race/ethnicity, breast volume, and comorbidities, only the comorbidity of DM was found to be significantly associated with moderate or pronounced scarring (P = 0.046). Of the tumor-dependent variables examined, no correlation was found between tumor location and extent of scarring, but a significant correlation was found between tumor location and extent of postoperative mammary retraction (P = 0.007). Of the treatment-dependent variables examined, a significant relationship was found between the type of incision and extent of scarring (P = 0.008). Among those patients with only subtle, almost absent scarring, a significant percentage had undergone periareolar incision; among those with slight scarring, a significant percentage had undergone arched incision; and among those with moderate or pronounced scarring, a significant percentage had undergone radial incision. These results indicate that periareolar incision yields the best aesthetic outcome and radial incision the worst. Among the treatment-dependent variables examined, use of CT was found to yield worse aesthetic outcomes than use of HT at a statistical level (P = 0.073) that almost reached significance (P = 0.05).
As RT is a fundamental component of conservative treatment of breast cancer, many studies have evaluated its effects on aesthetic outcomes12,28. While these studies all identified conventional RT as an isolated factor associated with unfavorable aesthetic outcomes, particularly when performed preoperatively26,28, the current study was the first to examine whether IORT is associated with specific aesthetic outcomes. Comparison of the 71.2% of patients who had undergone conventional RT with the 8.8% who had undergone IORT indicated no significant differences in the extent of scarring in these 2 groups. This finding indicates that administration of a single dose of 21 Gy of radiation, per IORT, and administration of 30 sessions of a total dose of 55 Gy of radiation, per conventional RT, affects the extent of scarring to the same extent. Regarding patient morphology, Clough et al.26 suggested a relationship between unfavorable cosmetic results and the existence of very little residual breast volume, and Taylor et al.9, among many other authors, found that cosmetic results decrease in proportion to resected mammary volume. As breast volume is a subjective measure, the initial estimations made by the observing doctors in this study were confirmed by other clinicians, and the estimated breast volume values subsequently evaluated to determine whether they corresponded with the extent of breast ptosis, a more objective measure. A significant relationship was found between the extent of ptosis and breast volume (P < 0.001), indicating that ptosis should be measured in future evaluations as a means of increasing the reliability of the results.
The results of the analysis of the relationship between estimated preoperative breast volume and identification of the more aesthetically pleasing breast almost reached a level of significance (P = 0.052). Specifically, neither breast was considered more aesthetically pleasing when mammary volume was estimated at < 200 cc (i.e., small breasts), while the untreated breast was considered more aesthetically pleasing when mammary volume was estimated at 200 to 400 cc (a volume considered aesthetically ideal), likely because the volume became inadequate with surgery. In contrast, the treated breast was considered more aesthetically pleasing when mammary volume exceeded 400 cc, most likely because upon removal of breast tissue (together with the tumor mass, independently of the resected volume), the breast had a more aesthetically adequate volume, and was therefore considered more aesthetically pleasing when compared to the oversized contralateral breast (Figure 11).
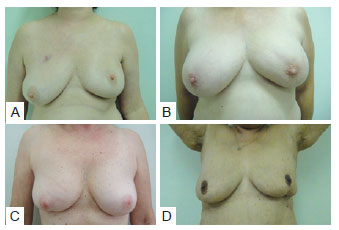
Figure 11 - In A and B, tumor resectioning in upper quadrant, presenting ptosis and consequently, the treated breast was considered more aesthetically pleasing. In C and D, tumor resectioning in lower quadrant, without ptosis alterations and, consequently, the treated breast was considered more aesthetically worst.
Curiously, no significant relationship was found between the use of mammary implants and identification of the more aesthetically pleasing breast, likely because the implant was used only to replace volume that had been resected, as the parameter for choice of implant is the weight of the removed surgical piece. While implantation improves symmetry, it does not necessarily improve overall aesthetic appearance. While several local flaps can be placed in the empty space left after removal of the tumor, it is often impossible to replace lost breast volume and give proper shape to the breast. Therefore, in many cases mammary remodeling should be provided when performing contralateral reducing mammoplasty as part of breast cancer treatment, as it is known that performing both surgical techniques at the moment of treatment may positively influence outcomes. Unfortunately, not all patients have access to these procedures10.
Examination of the relationship between aesthetic outcomes and several factors, including tumor-dependent factors, such as tumor site; patient-dependent variables, such as BMI and age; and treatment-dependent factors, such as type of RT and extent of shape change and of postoperative static or dynamic retraction, indicated that outcomes were significantly associated with only the extent of postoperative static and dynamic retraction (P = 0.012 and P = 0.026, respectively). While approximately 80% of patients with tumors in the upper quadrants had experienced change in CAP position, most patients with tumors in the lower quadrant did not, indicating a significant association between change in CAP position and quadrant (P = 0.002).
Classification of postoperative ptosis was most accurate in patients who had experienced an increase in the extent of ptosis. The classification was confirmed in the significant association found between change in CAP position and identification of the more aesthetically pleasing breast (P = 0.001), as 90% of patients showed an improvement in the position of the CAP; this can be easily explained by the fact that the CAP ascended as a result of resection of tissue above it.
In accordance with the literature10 regarding the importance of the correlation between extent of ptosis and outcomes, patients with pronounced ptosis (> 3 cm of the submammary groove) were found to be more satisfied with the outcome after the repairing procedure had been performed compared to those with less pronounced ptosis (< 1 cm). This finding indicates that when surgery is performed in the upper quadrants (particularly the QSM), resection of the tissue along the line between the sternal furcula and the areola will automatically elevate and better position the CAP in a manner similar to the "point A of Pitanguy," the highest point used in marking in reducing mammoplasties, equivalent to the ideal position of the CAP29. It is also clear, even if not significant, that performing surgery in the lower quadrant is very likely to have a negative impact on breast shape.
The study results suggest that when the tumor is localized in the lower quadrants (QIL, QIM, or UQI) and if the breast is not remodeled, as in the cases described in this study, the CAP will remain at the same height and the breast will partially lose shape, giving the impression that is a worsening of ptosis, i.e., a relative ptosis, since there was no change in the height of the CAP but only in the volume of the lower pole that caused the CAP to be in a position near the caudal limit of the breast. Clough et al.8 agree that patients with tumors in the lower quadrants are the primary, although not only, candidates for surgery accompanied by mammary remodeling instead of only primary closure of the surgical field.
All the results suggest that in the absence of retraction, mammary volume will decrease when the tumor is resected in a larger-than-ideal breast, elevating the CAP position in a manner associated with improvement of ptosis, thus making the treated breast more aesthetically pleasing compared to the healthy contralateral breast in a significant number of cases. This suggestion alone constitutes a reasonable indication for performing contralateral mammoplasty. Although the literature states that it is not uncommon for the surgically treated breast to be more aesthetically pleasing than the healthy breast, few studies describe the need to operate on the contralateral breast as part of breast cancer treatment30. Ideally, conservative breast surgery should not result in asymmetry or residual deformity, a fact not mentioned in most studies7,10,31. The unfavorable unaesthetic results of conservative breast surgery are invariably related to differences in the shape and volume of the breasts. According to Bajaj et al.30, the percentage of asymmetry resulting from segment-based surgery and primary closure of the surgical margins may reach 35%, making it an important negative factor in the aesthetic evaluation of the results. However, even a high degree of asymmetry may not be perceived during the intraoperative period, only becoming noticeable with time, particularly during healing or after RT32.
According to the Moro and Ciambellotti classification27, only 18 patients obtained excellent outcomes, meaning that 48 had experienced at least one negative result, whether change in CAP position, volume change, or obvious retraction. It is important to note that the surgically treated breast was considered more aesthetically pleasing than the healthy breast in 40% of the patients who had obtained satisfactory outcomes and, surprisingly, 70% who had obtained poor outcomes. These results suggest that when using the Moro and Ciambellotti classification27, a poor outcome indicates that surgery resulted in asymmetry, lack of volume, and/or inadequate CAP position, and does not mean that the surgery itself was inadequate-on the contrary, as the surgically treated breast was considered more aesthetically pleasing. These results thus lead to the conclusion that in a great number of cases, the quality of the surgery was good despite the resulting asymmetry, which could have been improved if the contralateral breast had been made more symmetrical.
The importance of performing mammary remodeling at the time of tumor resectioning was reinforced by Clough et al.'s26 study of aesthetic sequelae after conservative surgery in 3 groups of patients. One group was composed of patients who had undergone adequate breast surgery and required only symmetrization to improve aesthetics. At the other extreme, one group was composed of patients with clear breast deformities, including retractions and significant volume and shape deficits. Treating this group required total resectioning of the remaining mammary tissue-mastectomy-followed by total breast reconstruction using more complex techniques, such as distant flap manipulation. Although the need for a surgical approach was evident in this group, the patients were reluctant to undergo surgery because mastectomy had not been initially indicated as part of their breast cancer treatment. In such groups, it is important to alert patients to the possibility of poor outcomes and obtain their permission to perform mastectomy because performing mastectomy, including skin-sparing mastectomy, with immediate reconstruction ultimately leads to better outcomes and, most likely, fewer complications. The last group posed the most significant treatment challenge, being composed of patients who had obtained inadequate surgical outcomes. The primary challenge was that the conservatively treated breast had invariably been subjected to RT, scarring the tissue and making it fibrous, and thus unpredictable in terms of its reaction to future surgical manipulation. In this group, the results were not encouraging, as indicated by a high complication index in most cases.
Several studies have indicated that patients with predominantly fatty breasts may experience late complications, such as steatonecrosis and loss of breast volume, that result in asymmetrical reconstruction. In these patients, the defect resulting from oncological surgery should be corrected in manner that leaves the treated breast larger than the contralateral breast to make it possible to reduce the differences in the future. An outcome considered very good in the immediate postoperative period may, after RT, become compromised due to fibrosis and retraction over the remaining breast tissue7.
Although IORT was found to yield aesthetic results regarding scarring comparable to those of conventional RT in this study, the manner in which the irradiated breast tissue will react to future surgical manipulation is unknown, as the patients were not followed up by our institute. Concerning the psychological aspects of patients, Homberg et al.33 identified excessive aesthetic concerns prior to surgery and/or excessive valorization of the breasts as an aspect of body image as predictors of poor aesthetic prognosis. However, such predictors must be identified with the utmost caution.
Approaching cancer using mammoplasty techniques may, besides improved aesthetic appearance, offer as advantages technical facilitation of a broad surgical field and better outcomes, including scarring to only the slight extent that occurs in plastic surgery and a resected piece larger than that when using techniques aiming at simple primary closure of the surgical field. Despite increasing surgical time, contralateral breast symmetrization provides additional tissue for histopathological analysis and significantly improves aesthetic results in the immediate postoperative period34. However, popularization of the so-called oncoplastic technique is increasingly being associated with oncological teams, leading it to lose popularity among plastic surgeons and the emergence of conflicts of interests in selected cases when, by resecting enough tissue to allow adequate oncological analysis, the breast becomes deformed or too small. As postulated by Cardoso et al.24, resectioning of large quantities of tissue may result in poorer aesthetic outcomes. In these situations, a team composed of an oncological surgeon and an associated plastic surgeon is in the best interest of the patient26.
Collaboration between the mastologist and a plastic surgeon may also increase the therapeutic efficacy of the overall treatment by reducing costs through the provision of a single surgical procedure rather than multiple procedures performed by different specialists. In the same manner, collaboration can decrease the psychic morbidity associated with undergoing multiple surgical operations. These aspects have been widely accepted by patients, many of whom have seen such collaboration as an opportunity to correct and/or improve the appearance of their breasts20,34. While emphasizing the advantages of collaboration in the surgical treatment of breast cancer, this study, as has others, avoided using the term oncoplasty, as its use may suggest the participation of professionals not qualified in plastic surgery, therefore compromising the final results10.
CONCLUSION
In the conservative treatment of breast cancer, remodeling of residual mammary tissue and performance of contralateral mammoplasty immediately after tumor removal is ideal. However, provision of treatment that provides better results is often not possible. In such cases, the operative wound is primarily closed by simple closure of the surgical margins, frequently leading to asymmetry, a result that alone can yield unsatisfactory aesthetic results. Nevertheless, in some cases, mainly in patients who have large breasts and/or have undergone resectioning of the upper quadrants, factors that improve ptosis and appearance, the surgically treated breast is more aesthetically pleasing than the healthy breast despite the asymmetry. However, the converse has also been observed, with patients who have undergone resectioning of the lower quadrants often being left with a misshapen and aesthetically unpleasing treated breast. Therefore, when the tumor is located in the upper quadrants, contralateral mammoplasty for symmetrization, even if performed at a later period, is ideal, whereas tissue remodeling, performed immediately in anticipation of subsequent RT (and associated with contralateral mammoplasty, if necessary), may be required when the tumor is located in the lower quadrants. Regarding the factors that predict the best aesthetic results, the use of periareolar incision was found to decrease the extent of scarring whereas the presence of the comorbidity of DM and the use of CT were found to increase it. The outcomes of IORT, a more rapid and less exhausting means of RT compared to conventional RT, were shown to be comparable to those of conventional RT regarding aesthetic appearance of the breast and extent of scarring.
REFERENCES
1. Instituto Nacional do Câncer, Ministério da Saúde. Estimativa 2010 Incidência de câncer no Brasil. Disponível em:
2. American Cancer Society. Cancer facts & figures 2011. Atlanta: American Cancer Society, 2011. Disponível em:
3. Baldwin LM, Taplin SH, Friedman H, Moe R. Access to multidisciplinary cancer care: is it linked to the use of breast-conserving surgery with radiation for early-stage breast carcinoma? Cancer. 2004;100(4):701-9.
4. Veronesi U. New trends in the treatment of breast cancer at the Cancer Institute of Milan. AJR Am J Roentgenol. 1977;128(2):287-9.
5. Veronesi U, Banfi A, Saccozzi R, Salvadori B, Zucali R, Uslenghi C, et al. Conservative treatment of breast cancer. A trial in progress at the Cancer Institute of Milan. Cancer. 1977;39(6 Suppl):2822-6.
6. Veronesi U, Salvadori B, Luini A, Banfi A, Zucali R, Del Vecchio M, et al. Conservative treatment of early breast cancer. Long-term results of 1232 cases treated with quadrantectomy, axillary dissection, and radiotherapy. Ann Surg. 1990;211(3):250-9.
7. Almeida Júnior GL, Macedo JLS, Borges SZ, Souza AO, Henriques FAM, Suschino CMH, et al. Reconstrução mamária imediata após cirurgia conservadora do câncer de mama. Rev Soc Bras Cir Plást. 2007;22(1):10-8.
8. Clough KB, Thomas SS, Fitoussi AD, Couturaud B, Reyal F, Falcou MC. Reconstruction after conservative treatment for breast cancer: cosmetic sequelae classification revisited. Plast Reconstr Surg. 2004;114(7):1743-53.
9. Taylor ME, Perez CA, Halverson KJ, Kuske RR, Philpott GW, Garcia DM, et al. Factors influencing cosmetic results after conservation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 1995;31(4):753-64.
10. Webster RS. Avaliação da satisfação de pacientes submetidas à reconstrução mamária pós-setorectomia e simetrização mamária contralateral imediata. Rev Soc Bras Cir Plást. 2010;25(1):85-91.
11. Tostes ROG, Andrade Júnior JCCG, Amorim WC, Neves LJVA, Avelar LET, Silva LCR, et al. Reconstrução mamária nos quadrantes inferiores após quadrantectomias. Rev Bras Cir Plást. 2010;25(2):309-16.
12. Veronesi U, Luini A, Del Vecchio M, Greco M, Galimberti V, Merson M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med. 1993;328(22):1587-91.
13. Roelstraete A, Van Lancker M, De Schryver A, Storme G. Adjuvant radiation after conservative surgery for early breast cancer. Local control and cosmetic outcome. Am J Clin Oncol. 1993;16(4):284-90.
14. Volterrani F, Aldrighetti D, Bolognesi A, Di Muzio N, Reni M, Veronesi P, et al. Analysis of the results of 264 cases of small breast carcinoma treated with conservative surgery and radiotherapy. Radiol Med. 1991;82(3):322-7.
15. Orecchia R, Ciocca M, Lazzari R, Veronesi P, Petit JI, Veronesi U, et al. Intraoperative radiation therapy with electrons (ELIOT) in early-stage breast cancer. Breast. 2003;12(6):483-90.
16. Veronesi U, Gatti G, Luini A, Intra M, Ciocca M, Orecchia R, et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery. Arch Surg. 2003;138(11):1253-6.
17. Luini A, Orecchia R, Gatti G, Intra M, Ciocca M, Veronesi U, et al. The pilot trial on intraoperative radiotherapy with electrons (ELIOT): update on the results. Breast Cancer Res Treat. 2005;93(1):55-9.
18. Orecchia R, Ciocca M, Tosi G, Franzetti S, Luini A, Veronesi U, et al Intraoperative electron beam radiotherapy (ELIOT) to the breast: a need for a quality assurance programme. Breast. 2005;14(6):541-6.
19. Veronesi U, Orecchia R, Luini A, Galimberti V, Zurrida S, Intra M, et al Intraoperative radiotherapy during breast conserving surgery: a study on 1,822 cases treated with electrons. Breast Cancer Res Treat. 2010;124(1):141-51.
20. Frasson AL, Zerwes FP, Braga AP, Barbosa FS, Koch HA. Intraoperative radiotherapy in the conventional linear accelerator room for early breast cancer treatment: an alternative choice in developing countries. J Exp Clin Cancer Res. 2007;26(3):379-84.
21. Cordeiro PG. Breast reconstruction after surgery for breast cancer. N Engl J Med. 2008;359(15):1590-601.
22. Acea-Nebril B, López S, Cereijo C, Bazarra A, Pais P, Gómez C, et al. Impact of conservative oncoplastic techniques in a surgery program for women with breast cancer. Cir Esp. 2005;78(3):175-82.
23. Mosahebi A, Ramakrishnan V, Gittos M, Collier J. Aesthetic outcome of different techniques of reconstruction following nipple-areola-preserving envelope mastectomy with immediate reconstruction. Plast Reconstr Surg. 2007;119(3):796-803.
24. Cardoso MJ, Cardoso J, Santos AC, Vrieling C, Liljegren G, Oliveira MC, et al Factors determining esthetic outcome after breast cancer conservative treatment. Breast J. 2007;13(2):140-6.
25. Acea Nebril B. "Scarless" surgery in the treatment of breast cancer. Cir Esp. 2010;87(4):210-7.
26. Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg. 2003;237(1):26-34.
27. Moro G, Ciambellotti E. Evaluation of the esthetic results of conservative treatment of breast cancer. Tumori.1993;79(4):258-61.
28. Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124(2):395-408.
29.Pitanguy I. Evaluation of body contouring surgery today: a 30-year perspective. Plast Reconstr Surg. 2000;105(4):1499-516.
30. Bajaj AK, Kon PS, Oberg KC, Miles DA. Aesthetic outcomes in patients undergoing breast conservation therapy for the treatment of localized breast cancer. Plast Reconstr Surg. 2004;114(6):1442-9.
31. Garusi C, Petit JY, Rietjens M, Lanfrey E. Role of plastic surgery in the conservative treatment of breast cancer. Ann Chir Plast Esthet. 1997;42(2):168-76.
32. Waljee JF, Hu ES, Ubel PA, Smith DM, Newman LA, Alderman AK. Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. J Clin Oncol. 2008;26(20):3331-7.
33. Holmberg L, Zarén E, Adami HO, Bergström R, Burns T. The patient's appraisal of the cosmetic result of segmental mastectomy in benign and malignant breast disease. Ann Surg. 1988;207(2):189-94.
34. Pedron ML, Alves MR, Menk C. Sistematização em mamoplastia oncológica. Rev Soc Bras Cir Plást. 2001;16(3):47-60.
35. Asgeirsson KS, Rasheed T, McCulley SJ, Macmillan RD. Oncological and cosmetic outcomes of oncoplastic breast conserving surgery. Eur J Surg Oncol. 2005;31(8):817-23.
36. Triedman SA, Osteen R, Harris JR Factors influencing cosmetic outcome of conservative surgery and radiotherapy for breast cancer. Surg Clin North Am. 1990;70(4):901-16.
37. Heil J, Czink E, Golatta M, Schott S, Hof H, Jenetzky E, et al. Change of aesthetic and functional outcome over time and their relationship to quality of life after breast conserving therapy. Eur J Surg Oncol. 2011; 37(2):116-21.
38. Heil J, Dahlkamp J, Golatta M, Rauch G, Cardoso MJ, Sohn C, et al. Aesthetics in breast conserving therapy: do objectively measured results match patients' evaluations? Ann Surg Oncol. 2011;18(1):134-8.
39. Santanelli F, Paolini G, Campanale A, Longo B, Amanti C. The "Type V" skin-sparing mastectomy for upper quadrant skin resections. Ann Plast Surg. 2010;65(2):135-9.
40. Cardoso MJ, Cardoso JS, Wild T, Krois W, Fitzal F. Comparing two objective methods for the aesthetic evaluation of breast cancer conservative treatment. Breast Cancer Res Treat. 2009;116(1):149-52.
41. Pusic AL, Chen CM, Cano S, McCarthy C, Collins ED, Cordeiro PG. Measuring quality of life in cosmetic and reconstructive breast surgery: a systematic review of patient-reported outcomes instruments. Plast Reconstr Surg. 2007;120(4):823-39.
42. Benoit L, Franceschini C, Margarot A, Arnould L, Fraisse J, Cuisenier J. Conservative surgery in breast cancer: combination skin incision for a better cosmetic result. Ann Chir. 2004;129(5):310-2.
43. Cordeiro PG, Pusic AL, Disa JJ, McCormick B, VanZee K. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113(3):877-81.
Plastic surgeon at the Tournieux Plastic Surgery Clinic, full member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery), São Paulo, SP, Brazil.
Tatiana Tourinho Tournieux
Rua Guaninas, 68 - Jd. Aeroporto
São Paulo, SP, Brazil - CEP 04632-000
E-mail: ttournieux@hotmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: June 5, 2011
Article accepted: November 11, 2011
Study conducted at the Tournieux Plastic Surgery Clinic, São Paulo, SP, Brazil.
Study presented for promotion to a full member of SBCP.
Study awarded with the Nemer Chidid Prize 2011.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter