INTRODUCTION
Chest wall tumors are relatively uncommon, representing 1 to 2% of all neoplasms
and approximately 5% of thoracic neoplasms. Primary tumors of the chest wall are
rare and include a large group of neoplasms that can arise not only from bones
or cartilage of the chest wall but also from associated subcutaneous tissue of
muscles and blood vessels1.
Primary malignant tumors of the chest wall account for less than 1% of all
neoplasms and include a wide variety of bone and soft tissue lesions.
Chondrosarcomas represent 20% of primary tumors of the chest wall, with 80%
originating in the ribs and 20% in the sternum. Occurrence in the chest wall is
rare, representing 8% of all sarcomas1.
The treatment of choice is resection with wide margins and, when invasion of
deep structures occurs, thoracotomy is indicated2, 3, 4.
This study aimed to describe the clinical presentation, method of preoperative
planning and surgery, and perioperative management of giant sternal
chondrosarcoma. Sternectomy to treat malignant tumors results in large defects
in the bone framework and soft tissues, causing deformity and paradoxical
movement of the chest wall and making thoracic reconstruction very
important5. The reconstruction of the
bone framework is performed with synthetic materials providing stability and is
followed by the reconstruction of soft tissues with well-vascularized muscular,
musculocutaneous, or random local flaps5,
6, 7, 8, 9, 10, 11.
CASE REPORT
Male, 53 years old, born and living in Fortaleza (CE), admitted to the thoracic
surgery service at Hospital de Messejana, in 2021. Six months ago he presented
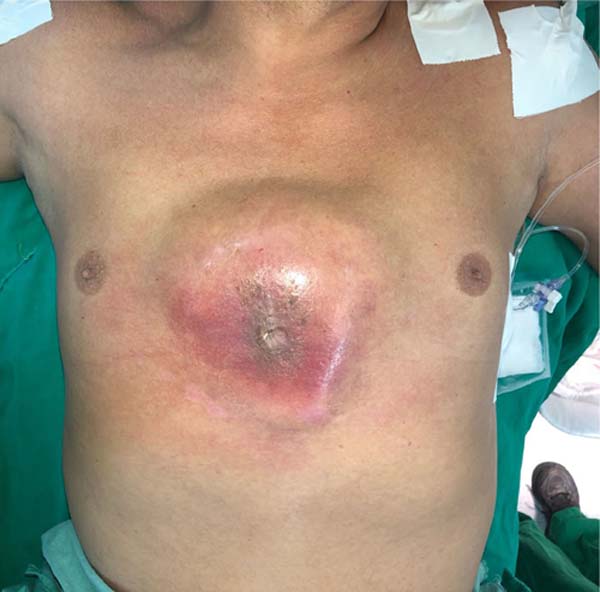
with a bulging sternum (Figure 1) with mild
local pain and a progressive increase in the lesion. He developed dyspnea on
exertion over the last month. He reported weight loss of around 10 kg in the
last six months.
Figure 1 - Initial presentation of the patient.
Figure 1 - Initial presentation of the patient.
On physical examination, he was in good general condition, anicteric, acyanotic,
afebrile, eucardial, and eupneic. Pulmonary and cardiac auscultation was normal,
and abdominal palpation was innocent. From complementary exams, the chest
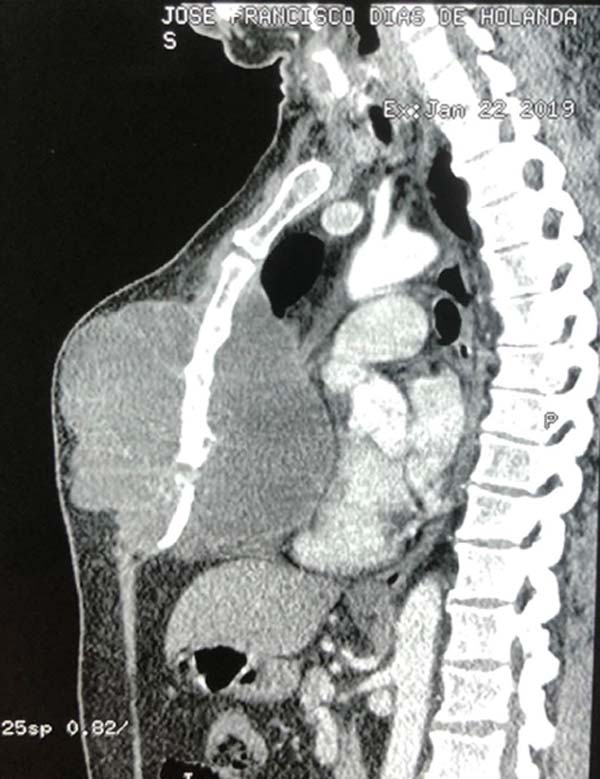
tomography (Figure 2) showed an expansive
and septate formation compromising the thoracic wall and with significant
involvement of the sternal body, with foci of osteolysis, measuring 15.3 ×
13.8cm, insinuating itself into the region intrathoracic and determining
significant posterior rejection of the heart, without signs of pericardial
invasion.
Figure 2 - Tomography of the tumor lesion with mediastinal invasion
preoperatively.
Figure 2 - Tomography of the tumor lesion with mediastinal invasion
preoperatively.
A bone scintigraphy was performed, which demonstrated diffuse irregularity in the
uptake of the drug in the sternum. The echocardiogram demonstrated a mediastinal
mass compressing the right heart cavities, mild pericardial effusion, and an
ejection fraction of 70%. An incisional biopsy of the lesion was performed, the
anatomopathological examination of which revealed a low-grade cartilaginous
neoplasm, suggestive of osteochondroma, which could not be completely
distinguished from grade 1 chondrosarcoma.
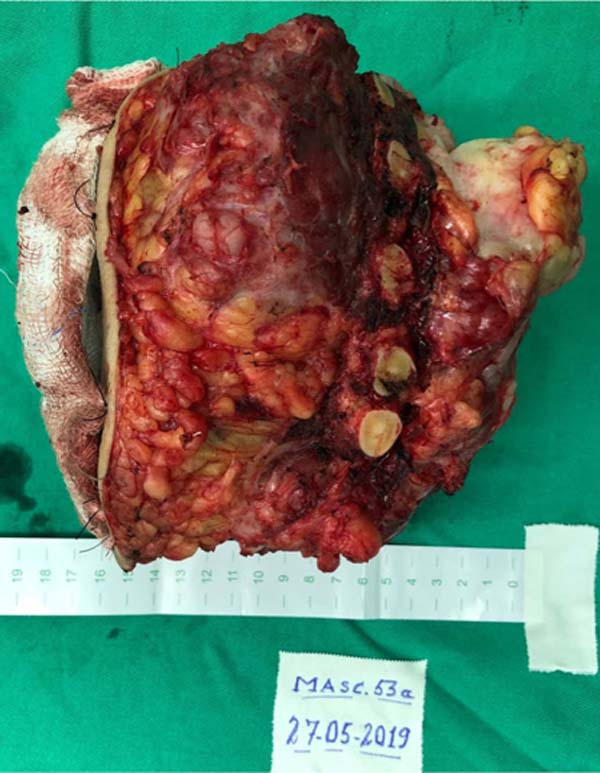
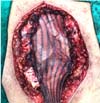
The patient underwent surgery, with complete resection of the tumor (Figure 3) by partial sternectomy en bloc
with cartilage from the 3rd to 10th costal arches, and preservation of the
manubrium (Figure 4). Reconstruction of
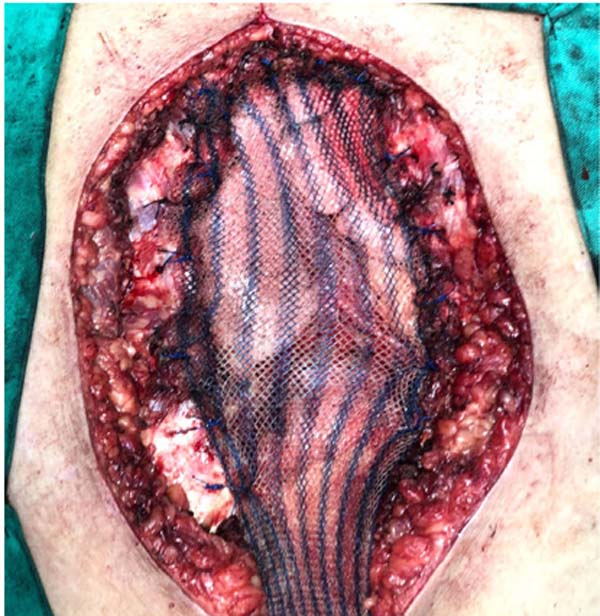
the chest wall was performed with double polypropylene mesh (Figure 5) and bilateral pectoral flaps as
described by Starzynski for the correction of large defects in the chest wall,
with the advent of the plastic surgery service of the reference hospital. The
final histopathological diagnosis was grade II chondrosarcoma, with a
microscopically compromised margin.
Figure 3 - Resected tumor lesion, aspect upon referral to
histopathology.
Figure 3 - Resected tumor lesion, aspect upon referral to
histopathology.
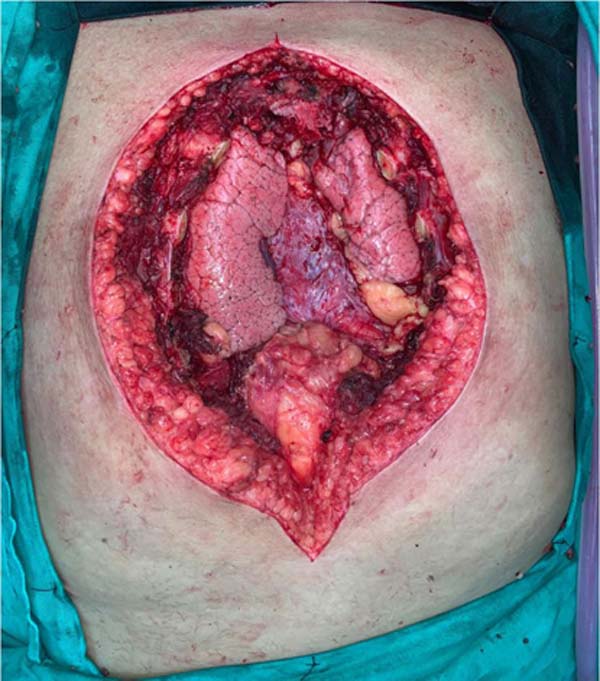
Figure 4 - Intraoperative image after removal of tumor mass showing exposed
mediastinum.
Figure 4 - Intraoperative image after removal of tumor mass showing exposed
mediastinum.
Figure 5 - Marlex mesh used as a form of additional protection to the
mediastinum.
Figure 5 - Marlex mesh used as a form of additional protection to the
mediastinum.
The patient underwent adjuvant chemotherapy and radiotherapy and evolved without
signs of tumor recurrence until the 9th-month post-surgery, when he underwent a
chest computed tomography scan which showed only findings compatible with
surgical manipulation, thickening of the subcutaneous tissue, and skin
retraction. In this case, reconstruction of the chest wall without the use of
bone cement or metallic prostheses is noteworthy, with good oncological,
aesthetic, and functional results (Figure 5). However, it is known that primary bone chondrosarcomas, hyaline
cartilage neoplasms, tend to show slow progression and high recurrence,
especially when surgical resection margins are not adequate, measuring at least
four centimeters on all sides.
DISCUSSION
Chondrosarcomas represent approximately 30% of primary malignant bone neoplasms,
the most common being those of the anterior chest wall. This tumor most commonly
occurs between the third and fourth decades of life and is relatively uncommon
in people under 20 years of age. Males are more affected3. Chondrosarcomas are lobulated neoplasms that can grow to
massive proportions and, consequently, can extend internally into the pleural
space or externally, invading muscle and adipose tissue of the chest wall.
Microscopically, findings range from normal cartilage to obvious malignant
changes.
Differentiating between chondroma and chondrosarcoma can be extremely
difficult3. A palpable mass in the
chest is the main symptom in approximately 80% of patients with a chest wall
tumor. Of these, 60% have associated pain2. Respiratory failure and hemothorax are rarer findings and are only
present when the tumors are very extensive4.
Imaging exams can be useful in suggesting the condition; however, a definitive
diagnosis requires a correlation between histology and radiology. Computed
tomography (CT) and magnetic resonance imaging (MRI) are good tests to
characterize the tumor and its extent. CT is superior to MRI for demonstrating
calcifications, while MRI is the exam of choice for evaluating the extent of the
tumor and its relationships with adjacent structures4.
Chest wall chondrosarcomas typically grow slowly and recur locally. If left
untreated, late metastases will occur. Complete control of the primary neoplasia
is the main determinant of survival. The objective of the first surgery should
be a resection wide enough to prevent local recurrence. This means getting a 4cm
margin on all sides. This approach results in the cure of approximately all
patients, resulting in a 10-year survival rate of 97%3.
Some authors propose that, as preoperative histological diagnosis is difficult,
wide resection should be performed in all cases of chest wall neoplasia5. Reconstruction of defects in the thoracic
costal framework and preservation of the manubrium. The patient presented
intraoperatively with the following defect in the rib cage (Figure 4). The reconstruction of the chest wall was
performed with double polypropylene mesh (Figure 5).
The reconstruction was defined with the plastic surgery service of the Hospital
de Messejana, where the following therapeutic options were postulated using the
algorithm proposed by the service concerning the reconstruction of the thoracic
framework:
1) To reconstruct the chest wall, we perform a muscle flap associated with skin
grafting (pectoralis major muscle); 2) Myocutaneous flaps: transverse rectus
abdominis muscle (TRAM) 3) vertical rectus abdominis muscle (VRAM); 4)
Association of TRAM with VRAM; 5) Latissimus dorsi muscle; 6) Fasciocutaneous
flaps from the region and bilateral pectoral flaps as described by Starzynski to
correct large defects in the chest wall with the advent of the plastic surgery
service of the reference hospital; 7) Free flaps.
Due to the degree of vascular compromise in the surgical resection, which
required the interruption of bilateral blood flow from the internal mammary
arteries, an arc of rotation with the latissimus dorsi muscle was not achieved.
The fasciocutaneous flap proposed by Starzynski (RASP- Rotation-advancement
split pectoralis) was suggested, a musculocutaneous flap based on the pectoralis
major muscle (type 5 Mates-Nahai), with bilateral ligation of the dominant
pedicles (thoracoacromial) and using the somersault flap. based on its
vascularization with intercostal accessory pedicles to cover the thoracic
framework defect at the sternal level (external absent and its topography
covered with polypropylene mesh).
Coverage of the skin defect was based on a wide sliding fasciocutaneous flap. The
fasciocutaneous flap was anchored with adhesion points described by Baroudi
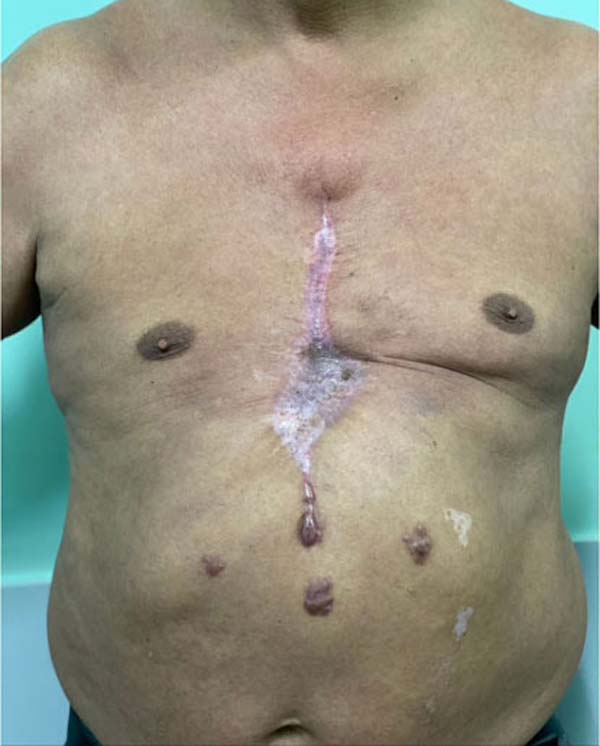
(Figure 6) and the patient developed
mild epidermolysis in the part downstream of the bilateral blood supply (edges
of the flap in the central region), which was treated conservatively, excellent
evolution was achieved (Figure 7).
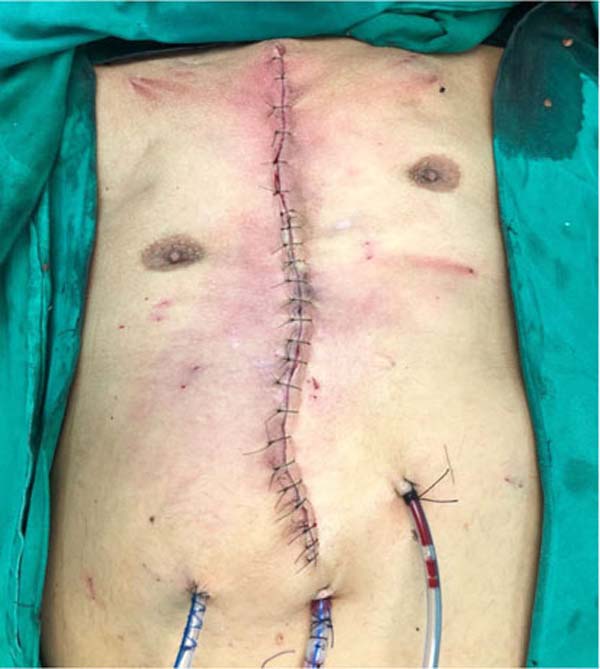
Figure 6 - Final appearance after reconstruction with Marlex mesh plus
muscle flaps and skin flaps anchored by Baroudi stitches.
Figure 6 - Final appearance after reconstruction with Marlex mesh plus
muscle flaps and skin flaps anchored by Baroudi stitches.
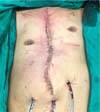
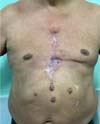
Figure 7 - Final appearance after surgical procedure.
Figure 7 - Final appearance after surgical procedure.
The final histopathological diagnosis was grade II chondrosarcoma, with a
microscopically compromised margin. The patient underwent adjuvant chemotherapy
and radiotherapy and evolved without signs of tumor recurrence until the 9th
month post-surgery, when he underwent a chest computed tomography scan which
only showed findings compatible with surgical manipulation, and thickening of
the subcutaneous tissue.
CONCLUSION
Through the progressive presentation of the case when he sought the thoracic
surgery service in Messejana, Fortaleza-CE, the necessary work-up was performed
to stage the tumor lesion following the precepts of the existing literature. The
patient’s evolution was satisfactory and we obtained a survival result with an
important quality of life, according to the international literature consulted.
It was possible to return the patient to his home activities with stipulated
monitoring to detect possible tumor recurrences through imaging exams on
predetermined dates.
The result obtained, as well as the surgical proposal given the clinical picture
presented by the patient, corresponds to a challenge given the size of the
lesion, the need for free margins, and the topography of the lesion, but
achieved success despite adversities.
REFERENCES
1. Gonfiotti A, Salvicchi A, Voltolini L. Chest-Wall Tumors and
Surgical Techniques: State-of-the-Art and Our Institutional Experience. J Clin
Med. 2022;11(19):5516. DOI: 10.3390/jcm11195516
2. Walsh GL, Davis BM, Swisher SG, Vaporciyan AA, Smythe WR,
Willis-Merriman K, et al. A single-institutional, multidisciplinary approach to
primary sarcomas involving the chest wall requiring full-thickness resections. J
Thorac Cardiovasc Surg. 2001;121(1):48-60. DOI:
10.1067/mtc.2001.111381
3. Rascoe PA, Reznik SI, Smythe WR. Chondrosarcoma of the thorax.
Sarcoma. 2011;2011:342879. DOI: 10.1155/2011/342879
4. Wang L, Yan X, Zhao J, Chen C, Chen C, Chen J, et al. Expert
consensus on resection of chest wall tumors and chest wall reconstruction.
Transl Lung Cancer Res. 2021;10(11):4057-83. DOI:
10.21037/tlcr-21-935
5. Dai Z, Maihemuti M, Sun Y, Jiang R. Resection and reconstruction of
huge tumors in the chest wall. J Cardiothorac Surg. 2022;17(1):116. DOI:
10.1186/s13019-022-01877-9
6. Isaac KV, Elzinga K, Buchel EW. The Best of Chest Wall
Reconstruction: Principles and Clinical Application for Complex Oncologic and
Sternal Defects. Plast Reconstr Surg. 2022;149(3):547e-62e. DOI:
10.1097/PRS.0000000000008882
7. Sebayang ANO. Management of Chest Wall Tumors with Tahalele’s
Method: Review Article. J Heal Study Med. 2020;2:109-16. DOI:
10.36145/JHSM2020.14
8. Crowley TP, Atkinson K, Bayliss CD, Barnard S, Milner RH, Ragbir M.
The surgical management of sarcomas of the chest wall: A 13-year single
institution experience. J Plast Reconstr Aesthet Surg. 2020;73(8):1448-55. DOI:
10.1016/j.bjps.2020.02.036
9. Çitak N, Çelikten A, Metin M, Pekçolaklar A, Gürses A. Radical
resection of a giant recurrent chondrosarcoma of the anterior chest wall. Gen
Thorac Cardiovasc Surg. 2011;59(7):512-4.
10. Stanic V, Vulovic T, Novakovic M, Ristanovic A, Stamenovic D,
Cvijanovic V, et al. Radical resection of giant chondrosarcoma of the anterior
chest wall. Vojnosanit Pregl. 2008;65(1):64-8.
11. Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of
500 consecutive patients. Plast Reconstr Surg. 1996;98(5):804-10. DOI:
10.1097/00006534-199610000-00008
1. Hospital de Messejana, Fortaleza, CE, Brazil
Corresponding author: José Dalvo Maia
Neto Avenida Pintor Antonio Bandeira, 1500, ap 1001, Vicente Pizon, Fortaleza, CE, Brazil. Zip Code: 60182-292, E-mail:
dalvomaia@hotmail.com
Article received: October 16, 2023.
Article accepted: April 30, 2024.
Conflicts of interest: none.
Institution: Hospital de Messejana, Fortaleza, CE, Brazil.